At the Heart of a First R01 Grant
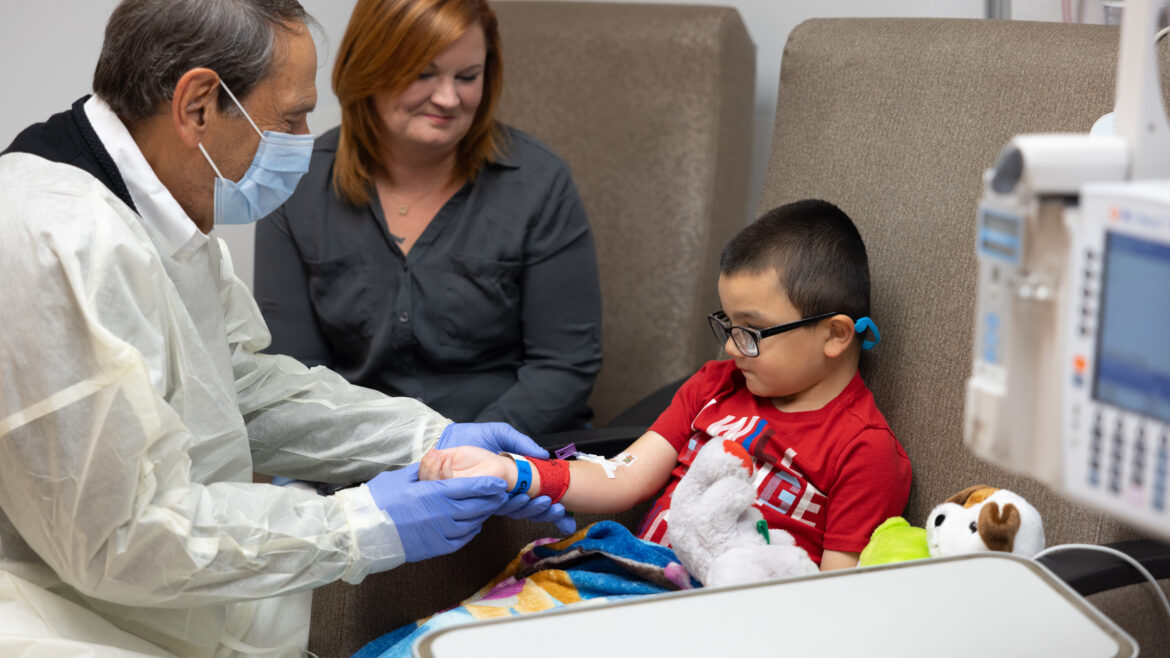
At the Heart of a First R01 Grant https://pediatricsnationwide.org/wp-content/themes/corpus/images/empty/thumbnail.jpg 150 150 Alaina Doklovic https://pediatricsnationwide.org/wp-content/uploads/2023/11/100923RH0019-e1699635391623.jpgAllison Bradbury, MS, PhD, is a principal investigator in the Center for Gene Therapy at Nationwide Children’s Hospital and an assistant professor in the Department of Pediatrics at The Ohio State University. She recently received her first R01 grant to develop novel therapeutic strategies for tubulin folding cofactor D (TBCD)-related developmental and epileptic encephalopathy. This…