Innovative Gene Therapy Approach for Treating Patients with Tuberous Sclerosis Type 2
Innovative Gene Therapy Approach for Treating Patients with Tuberous Sclerosis Type 2 https://pediatricsnationwide.org/wp-content/themes/corpus/images/empty/thumbnail.jpg 150 150 Lauren Dembeck Lauren Dembeck https://pediatricsnationwide.org/wp-content/uploads/2021/03/Dembeck_headshot.gif- January 11, 2024
- Lauren Dembeck
Tuberous sclerosis complex (TSC) is a devastating genetic disease that affects nearly 1 in every 5,500 newborns and approximately 2 million people worldwide. The disease is characterized by the formation of non-malignant tumors throughout multiple organs, including the kidney, lungs, eyes, and heart, but predominantly the brain. It is typically diagnosed in infants and young children, who frequently suffer morbidity due to neurologic complications. TSC is also one of the leading genetic causes of syndromic autism. Of various neurologic symptoms, severe and frequent seizures are among the most debilitating and in some cases fatal complications can result from hydrocephalus or from sudden unexpected death in epilepsy (SUDEP).

Mark Hester, PhD
“There is currently no cure for TSC. In the past decade, we’ve learned that seizure control is very important because it is a major determinant of cognitive outcomes in patients. However, even with some of the most potent antiseizure medications, only about 50% of patients have clinically relevant reductions in seizure frequency,” explains Mark Hester, PhD, who is a principal investigator in the Steve and Cindy Rasmussen Institute for Genomic Medicine at Nationwide Children’s Hospital. “When patients no longer respond to these antiseizure medications, resective surgery is an option, but this approach only offers a 50% chance of long-term seizure freedom. We need to address the root cause of the condition.”
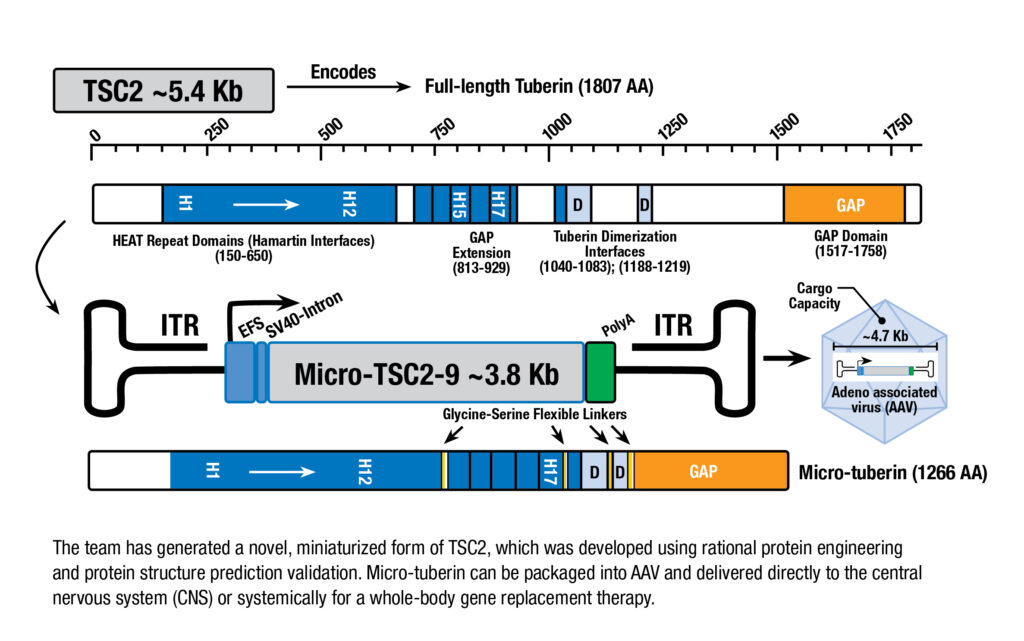
The most severe form of TSC, Type 2, are caused by mutations in the TSC2 gene, which encodes a protein called Tuberin. Together with other proteins, Tuberin forms a protein complex that regulates a critical cell signaling pathway involved in cell growth and metabolism. Dysfunction of this pathway leads to enlarged cells and tissue overgrowth.
Dr. Hester and colleagues have developed and are now testing a gene replacement therapy for TSC Type 2. The gene therapy uses adeno-associated viruses (AAV) to deliver a functional version of the gene to affected cells. AAVs have been use in the FDA-approved gene replacement therapies for treatment of spinal muscular atrophy and for certain patients with Duchenne muscular dystrophy, both of which were developed at Nationwide Children’s.
“AAV vectors have multiple advantages. They are safe, non-integrative, highly efficacious for gene delivery, and provide a potentially one-time curative treatment for patients,” says long-time colleague Craig McElroy, PhD, vice president of Pharmacokinetics at InfinixBio, an Ohio-based contract research organization. “However, these vectors have a limited cargo capacity, and the TSC2 gene is too large to be packaged into current vectors.”
To overcome this limitation, the team devised a novel strategy to replace the faulty TSC2 gene with a miniaturized version that when encoded still retains nearly the exact structure and function of the full-length Tuberin protein. To do so, they leveraged principles based on rational protein design and artificial intelligence-based protein structure prediction.
“TSC causes severe seizures, autism, brain tumors and other organ complications, such as kidney disease. As clinicians, we currently treat symptoms, but do not have a way to treat the underlying cause. The innovative approach Dr. Hester and his colleagues are taking with TSC2 has great potential to enable us to treat the whole person in a revolutionary way,” says Adam Ostendorf, MD, pediatric neurologist and medical director of the Epilepsy Surgery Program at Nationwide Children’s.
“One thing that is different about this gene therapy opportunity is the tremendous amount of effort that was put into creating the replacement gene,” shares Andrew Corris, PharmD, JD, a senior licensing associate in Nationwide Children’s Office of Technology Commercialization. “It was done in a very informed way that we typically would not see with other products. That gives me a lot of confidence in the potential efficacy of this product.”
The researchers have begun preliminarily testing several of their “micro-Tuberin” candidates and in vitro efficacy studies have shown robust proof-of-concept for further advancement.
“Gene therapy is a transformative treatment approach. The resources, the team science collaborative environment, and the proven track record of developing FDA-approved gene therapies at Nationwide Children’s allows us to be highly successful,” says Dr. Hester. “Considering Dr. McElroy’s background in structural biology and biochemistry and my expertise in neurobiology and gene therapy — this project would never have happened without forging a collaboration like this one.”
About the author
Lauren Dembeck, PhD, is a freelance science and medical writer based in New York City. She completed her BS in biology and BA in foreign languages at West Virginia University. Dr. Dembeck studied the genetic basis of natural variation in complex traits for her doctorate in genetics at North Carolina State University. She then conducted postdoctoral research on the formation and regulation of neuronal circuits at the Okinawa Institute of Science and Technology in Japan.
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/January 29, 2019







