Gene Therapy for the Masses?
Gene Therapy for the Masses? https://pediatricsnationwide.org/wp-content/uploads/2024/02/04-gideonrecievesgenetherapy-1024x683.jpg 1024 683 Katie Brind'Amour, PhD, MS, CHES Katie Brind'Amour, PhD, MS, CHES https://pediatricsnationwide.org/wp-content/uploads/2021/03/Katie-B-portrait.gif- February 27, 2024
- Katie Brind'Amour, PhD, MS, CHES
Long-lived financial and logistical hurdles make bringing new gene therapy products to market a major challenge. To help bring more of these medical miracles to fruition, experts across industry, regulatory review, science and medicine have begun to problem solve together.
With the approval of the gene therapy Kymriah® (tisagenlecleucel) in 2017, the Food and Drug Administration (FDA) kicked off a new era in medical therapy in the United States.
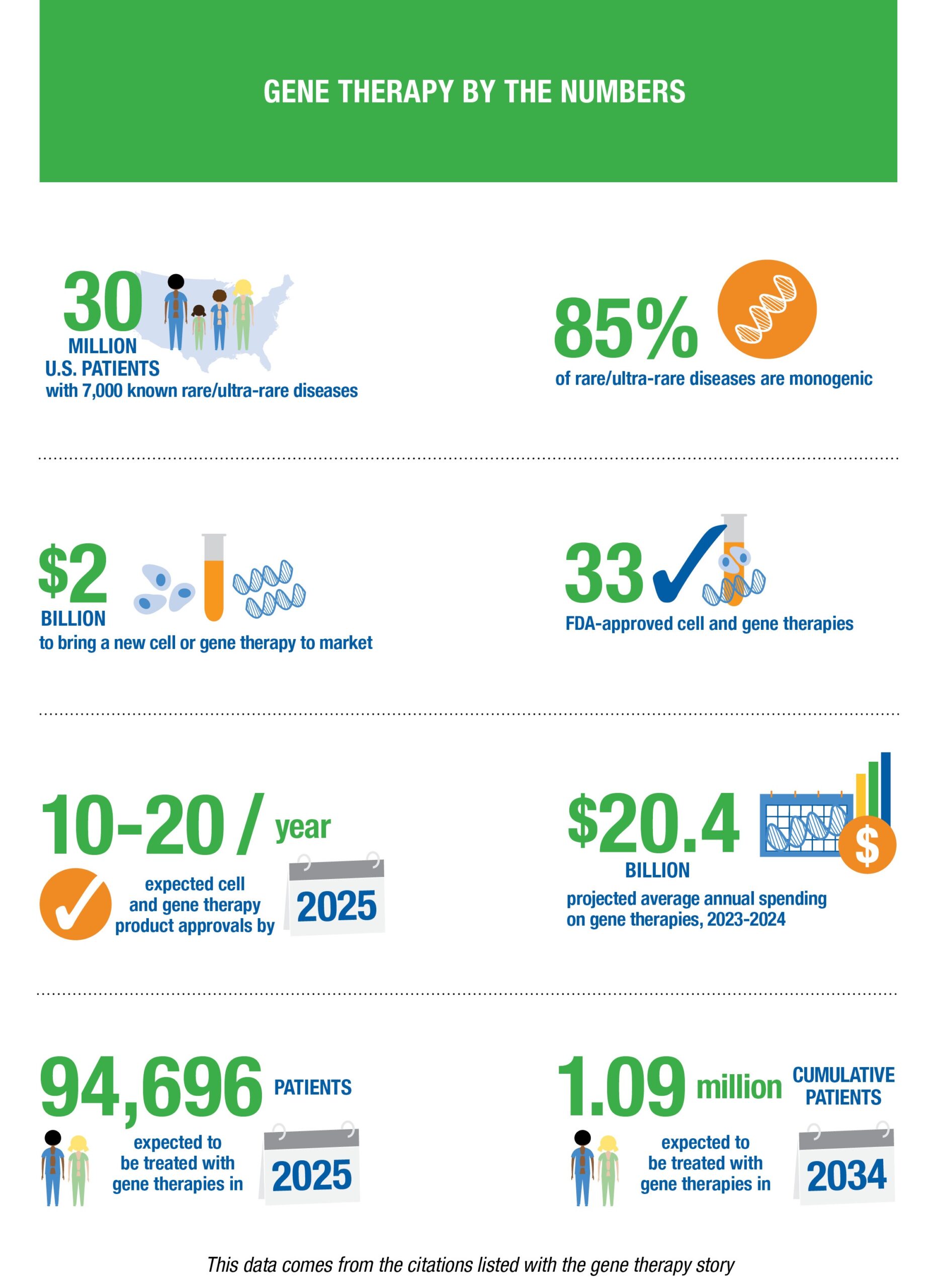
This watershed moment stoked an already raging fire in the cell and gene therapy industry. In the time since Kymriah’s entry to the market, another 33 cell and gene therapies have received approval, and more than 300 investigational new drug (IND) applications for gene therapies are now filed with the FDA each year.
As promising as these figures are, the fact remains that gene therapies are phenomenally expensive to develop and manufacture. On average, it costs nearly $2 billion to bring a new cell or gene therapy to market — a downside passed along to patients and payers, with the costliest therapy to date, Hemgenix®, priced at about $3.5 million per dose.
How can development and dosing at these costs be sustainable? Despite their tremendous potential to treat and cure disease, small markets and sky-high production costs leave many potential therapies unexplored, and thus, many patients untreated.
Thankfully, the field of gene therapy is full of experts pursuing solutions to the economic problems plaguing the industry. Together, they are actively seeking opportunities for efficiency in development, manufacturing, regulation and post-market policies to make it possible for more life-saving gene therapies to reach patients.
An Uphill Battle
The majority of the drug development market relies on medium- or large-scale production of medications purchased and used repeatedly by large numbers of people, which enables developers to recoup the costs of drug development and cover losses from failed segments of the portfolio. As science advances to the point of being able to treat hundreds of rare and ultra-rare diseases through single-dose therapies, a new framework for drug creation, production and reimbursement must emerge.
“We have licensed several gene therapy programs that have been returned to us, because companies don’t see them as commercially viable — there are so few patients that it doesn’t make strategic sense to proceed,” says Margaret Barkett, PhD, director of licensing for the Office of Technology Commercialization at Nationwide Children’s Hospital. Her team licensed two of the current FDA-approved gene therapies. “It’s a pity because often you have the IND, you have the gene therapy product manufactured and you’re ready to go, you just don’t have the funds to move it through the rest of the process.”
With gene therapy, the risk of each investment is amplified; potential market size (and thus potential profit) is typically small, but the research and development required to generate the therapy is at least as expensive as for any other drug.
These concerns become even more challenging when the patient population is so limited that the therapies cannot realistically or ethically be evaluated in comparison to natural history or placebo groups. The result is often insufficient or underwhelming data, even in situations with promising initial studies. This endangers investments, puts patients at risk, and forces regulatory agencies into difficult decisions between potential risks and rewards for patients.
Obtaining the necessary upfront capital is, therefore, an understandable challenge. If the drug makes it through\ trials at all, profit must be squeezed from only a few dozen doses per year, at a price tag that often raises objections from payers and the public. In addition, many gene therapies struggle to break into foreign markets due to high costs, further limiting market potential to very few high-income countries.
For companies manufacturing a therapy that treats only a few patients per year, covering overhead of running the facility for the remainder of the year is also problematic. Facilities that are not diversified and able to produce products for multiple therapies struggle to maintain the expensive staff and equipment. At least two gene therapy companies closed in autumn 2023 alone.
“Most small foundations and even researchers often severely underestimate what it will cost to get a gene therapy to trial.”
– Kevin Flanigan, MD, director of the Center for Gene Therapy in the Abigail Wexner Research Institute at Nationwide Children’s
“We try to be very, very clear about the escalating costs at different stages of a viral vector program,” says Dr. Flanigan. “Component One is discovery and lead candidate optimization work. Component Two triggers another big expense: the IND-enabling toxicology and dose-escalating work. Then, Component Three brings another order-of-magnitude change in costs: making the doses to give to an actual human.”
In cases where production does proceed to pivotal clinical trials or to market, companies face similar set-up costs regardless of the size of the batch being made. Whether the goal is to produce 10 doses or 10,000, purification processes, quality controls and verification of materials’ safety take similar time and money. Clinical good manufacturing processing (cGMP) facilities must also maintain other aspects of the facility regardless of batch size, such as single-use technologies, recycled air to avoid cross-contamination and regulatory requirements.
Regulatory complexity only adds to the expense, requiring analysis and review of each source material and step of the process — for each batch. The tests often deplete large portions of the manufactured product, leaving significantly less for dosing. When upfront investments allow, some companies opt to develop clinical-quality batches straight from the beginning of preclinical work in the hopes that a single batch will get them to market.
Regulatory agencies, researchers and contract development and manufacturing organizations (CDMOs) have been working to address some of the key causes of slow regulatory review: bespoke processes, variable controls and production methods, expensive materials and high-touch manufacturing techniques.

Regulatory Red Tape and Silver Linings
Regulatory challenges arise in part from the fact that cell and gene therapies are still the new kids on the pharmaceutical block. Each therapy, viral vector and production process is evaluated individually, even if significant portions of the process or source materials have already been reviewed and found sufficient for approved therapies.
“Prior to COVID, the FDA felt the process was the product, so if there was a change in cell lines or media, it meant starting over,” says Wade Macedone, CEO of Andelyn Biosciences, a CDMO working from concept to commercialization in viral vector production. “There is now concerted effort from the industry to show that the science and analytics are established — to address fear of the unknown that was driving costs and hindering speed and innovation. In the last 12 months or so, they’ve been starting to let industry solve problems with analytics, much more efficiently than by going back to the clinic.”
The FDA is working toward a more rapid and flexible review process across the board. Ideally, they want the field of gene therapy to get to a platform model — a shared system within a given viral vector, with the same production processes. Familiar, shared materials and processes then demand less attention with each new drug application. This “plug and chug” approach would subject just the new genetic payload and the clinical trial data to full scrutiny, rather than the entire operation.
“Honestly, if we’re going to try to get through thousands of diseases, we’re not going to get there the way we’re doing it right now,” said Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA, at the 2023 Technology Showcase sponsored by the Office of Technology Commercialization at Nationwide Children’s. “We need to figure out ways to leverage the things in gene therapy that facilitate us moving from on gene therapy to another. In other words, we need to stop treating them like small molecules or protein therapeutics.”
To this end, the Bespoke Gene Therapy Consortium (BGTC), funded by a private-public partnership called the Accelerating Medicines Program through the NIH, FDA and other organizations, funds platform innovations that can streamline gene therapy development. The program aims to create a protocol “playbook” that other researchers can use to develop gene therapies with less difficulty and expense.
The National Institute of Neurological Disorders and Stroke (NINDS) also funds Innovation Grants to Nurture Initial Translational Efforts (IGNITE), providing resources for neurotherapeutic agent characterization and in vivo efficacy studies. From there, the Ultra-rare Gene-based Therapy (URGenT) program funds promising therapies for ultra-rare monogenic neurological diseases in late preclinical through clinical trials.
To help streamline review and development efforts, the FDA announced the Support for clinical Trials Advancing Rare disease Therapeutics (START) Pilot Program. It will test a new way of communicating with drug developers, aiming to speed up the feedback and enable faster, more financially feasible development by reducing rework and costly waiting periods.
Most gene therapies under development also qualify for privileged FDA statuses, such as Priority Review. The review status comes as a “voucher” and can save 4+ months over standard review timelines. These can be sold for $100 million or more and offer another creative tactic some companies have used to fund gene therapy development.
Dr. Marks is also hopeful that global regulatory convergence can streamline the regulatory review and approval process for gene therapy candidates moving forward.
Finding Financial Answers
Gene therapy isn’t just focused on rare and ultra-rare diseases. There are many companies in the race to develop gene therapies for larger markets — everything from obesity and alcoholism to cancer have been considered candidates for novel gene therapies.
These indications, which have much larger annual markets than monogenic diseases, have the potential to fund discovery and development costs for ultra-rare gene therapies by making it easier to justify the expense of viral vector production.
“As we’re able to scale and meet needs of larger indications, that’s going to be a paradigm shift,” says Andrew Moreo, head of process development, plasmid and viral vector core facilities for Andelyn Biosciences. “The first time we get an indication that can cover a lot of people, that will start to offset costs.”
Risk-sharing agreements between academic institutions, manufacturers and funding organizations may also help ease the upfront burden and financial risk of producing these costly therapies for trials or markets. These approaches ensure no single investor takes a debilitating hit when a therapy fails.
“From a licensing perspective, we are trying to figure out ways to broker deals with partners to mitigate risk and share the cost of bringing these gene therapies to patients,” says Dr. Barkett.
Once a therapy does hit the market, paying for them to actually be delivered to patients is yet another challenge. Creative solutions are emerging for this problem as well, such as pooled insurance provider accounts that can be used to cover therapy for individuals in need, regardless of their insurer.
Thinking globally, lower-income countries could be an even better potential market than other high-income nations, both for patient benefit and for making it more financially feasible to run sizeable batches of gene therapy products that can be put to good use across a global population.
“In many parts of the world, people die of treatable diseases because they cannot get access to ongoing care,” says Macedone. “A one-and-done therapeutic dose could spare them a lifetime of morbidity or even early death due to lack of supportive care.”
With advancements in manufacturing and trends toward regulatory standardization, breakthroughs should bring with them a major rebound in capital investments — which will translate to greater access to gene therapy for many patients in need.
Developing Manufacturing and Scientific Solutions
Automation and simplification of the gene therapy production process may offer significant cost savings, similar to the hard-won cost-saving advancements achieved in the manufacturing of biologic therapies.
“Consolidating work into facilities such as Andelyn that can handle multiple products, increase standardization of processes and platforms and spread costs of goods across multiple different products can make the whole process more sustainable,” says Moreo. “We’re about 10 years behind the monoclonal antibody industry; once they settled on best practices for manufacturing and purification, that’s when you saw it take off and get efficient.”
Some of the latest innovations on the market include end-to-end robotic manufacturing, wherein customizable controls can be leveraged for the entire process to be completed in a clean room with no human intervention. Automation can also improve reproducibility and generate ongoing, informative data during live production, allowing teams to make adjustments as needed to reduce batch-to-batch variability.
Improvements in manufacturing efficiency would be all for naught, however, if therapies cannot prove their value in preclinical models and clinical trials.
Animal models for rare monogenic disorders are not readily available for most diseases. Furthermore, models developed with only limited transplants of human cells do not allow researchers to understand the specificity of a potential therapy, or its off-target effects. Although some new models for neurodegenerative diseases have been discovered, they can be costly and challenging to study.
More customizable emerging options include organoids, which are 3D tissue models of diverse cell populations derived from adult stem cells to mimic the cellular environment of a particular organ. These offer additional data about disease mechanisms as well as the potential safety and efficacy of a gene therapy approaching clinical trials. Understanding the therapy’s impact in human organoids helps bridge the gap between animal models or cell line studies and human trials. In time, patient-specific organoids treated with gene therapy could even be transplanted into patients to improve function of affected organs.
Even more exciting, body-on-a-chip models combine multiple different organoids (such as liver, heart, lungs, kidney and more) and additional induced pluripotent stem cells in a universal media. These are contained in a microfluidic platform with biophysical and chemical cues and stressors that mimic the body’s environment. This approach may allow scientists to understand how a gene therapy affects multiple organs and body tissues, as well as immunogenicity, genomic integration and biodistribution.
Other advancements for adeno-associated viral vector-based therapies in particular aim to improve the likelihood of therapy success and safety by improving screening for potential immunogenicity, studying off-target effects, immunostaining to determine tissue tropism, examine risks for genomic integration and improve tissue-specific toxicity knowledge.
Moreo explains that, in viral-mediated gene therapy, the challenges stem from having many different biologic products.
“Way down the road, we can expect things to evolve to nonviral or even synthetic, virus-like nanoparticles,” he says. “These not only carry a genetic payload but also target specific areas or organs, and that on-target specificity will be key.”
While these advances improve preclinical progress and data-gathering opportunities, little can be done about limited populations for clinical trials, which inherently limits knowledge about a therapy’s safety and efficacy.
To address this, the FDA has begun to issue advice on common challenges to gene therapy, including the draft guidance “Demonstrating Substantial Evidence of Effectiveness Based on One Adequate and Well-Controlled Clinical Investigation and Confirmatory Evidence,” which is expected to be the first of many guidance documents advising more standardized and acceptable approaches to small-population gene therapy development.
While statistical methods and careful preclinical investigations do not fully replace large-scale phase 3 trials, they attempt to make the best of a challenging situation by enabling approval and therapy access to desperate patients, despite data limitations.
The Coming Gene Therapy Revolution
With several decades of lessons under the belts of researchers, manufacturers and investors, gene therapy is primed to transform the future of medical therapy for millions of individuals.
Significant safety improvements allow new therapies to proactively avoid many concerns present in prior iterations. Researchers now more clearly understand , what developing a therapy entails, and more realistic expectations can be given to investors and hopeful families or funding organizations.
Nationwide Children’s Center for Gene Therapy recently established a Personalized Gene Therapy Committee, for example, to help align expectations and facilitate communication between researchers and investors — often private foundations, advocacy organizations or even individuals — as a new gene therapy is pursued. It bases the communication and review process on the fount of knowledge accumulated by researchers and technology transfer specialists during the hospital’s numerous gene therapy trials and licensing successes. The group also discusses opportunities to ensure access to research for populations without wealthy donors or active patient advocacy groups.
As the field faces its current challenges, the future is bright.
“Industry leaders are coming to the table to find solutions to the problem [of high costs],” says Macedone. “The community wants it, the government wants it, the scientists want it. Funding will come back, disease populations that can be treated will get larger, and we will get to the point that it’s as easy to get gene therapy as it is to take a dose of aspirin. It’s decades, if not a generation, off. But we’ll get there.”
More therapies will be developed and approved. More manufacturing and regulatory solutions will emerge. And more patients will be treated.
References:
- Sabatini MT, Chalmers M. The cost of biotech innovation: Exploring research and development costs of cell and gene therapies. Pharmaceutical Medicine, 2023;37:365–375.
- FDA. “FDA launches pilot program to help further accelerate development of rare disease therapies.” U.S. Food & Drug Administration. 29 Sept 2023. Accessed online at: FDA Launches Pilot Program to Help Further Accelerate Development of Rare Disease Therapies | FDA.
- Geurts MH, Clevers H. CRISPR engineering in organoids for gene repair and disease modelling. Nature Reviews Bioengineering, 2023;1:32–45.
- Ramamurthy RM, Atala A, Porada CD, Almeida-Porada G. Organoids and microphysiological systems: Promising models for accelerating AAV gene therapy studies. Frontiers in Immunology, 2022 Sep 26;13:1011143.
- Wong CH, Li D, Wang N et al. The estimated annual financial impact of gene therapy in the United States. Gene Therapy, 2023:30:761–773.
- FDA. “Statement from FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D., Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies.” U.S. Food & Drug Administration. 15 Jan 2019. Accessed online at https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics.
- FDA. “Approved cellular and gene therapy products.” U.S. Food & Drug Administration. 4 Dec 2023. Accessed online at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products.
- https://www.ninds.nih.gov/current-research/research-funded-ninds/translational-research/ultra-rare-gene-based-therapy-urgent-network
About the author
Katherine (Katie) Brind’Amour is a freelance medical and health science writer based in Pennsylvania. She has written about nearly every therapeutic area for patients, doctors and the general public. Dr. Brind’Amour specializes in health literacy and patient education. She completed her BS and MS degrees in Biology at Arizona State University and her PhD in Health Services Management and Policy at The Ohio State University. She is a Certified Health Education Specialist and is interested in health promotion via health programs and the communication of medical information.
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 28, 2014