An Expanded, Multicenter Look at Gene Therapy for Spinal Muscular Atrophy
An Expanded, Multicenter Look at Gene Therapy for Spinal Muscular Atrophy https://pediatricsnationwide.org/wp-content/uploads/2020/08/Cover-V3-Flat-CMYK-HEADER-FOR-WEB-1024x575.gif 1024 575 Mary Bates, PhD Mary Bates, PhD https://secure.gravatar.com/avatar/c6233ca2b7754ab7c4c820e14eb518c8?s=96&d=mm&r=g- August 25, 2020
- Mary Bates, PhD
New study confirms safety and efficacy in children under two years old.
In May 2019, the U.S. Food and Drug Administration (FDA) approved a gene replacement therapy for the inherited, progressive neuromuscular disease 5q-linked spinal muscular atrophy (SMA). Approval included all children with SMA under the age of two years; however, the gene therapy had only been studied in children aged up to 8 months.
Now, a new study discusses safety and early outcomes in a large cohort of SMA patients under the age of two years who were treated with gene therapy. The report is published in the journal Pediatrics.
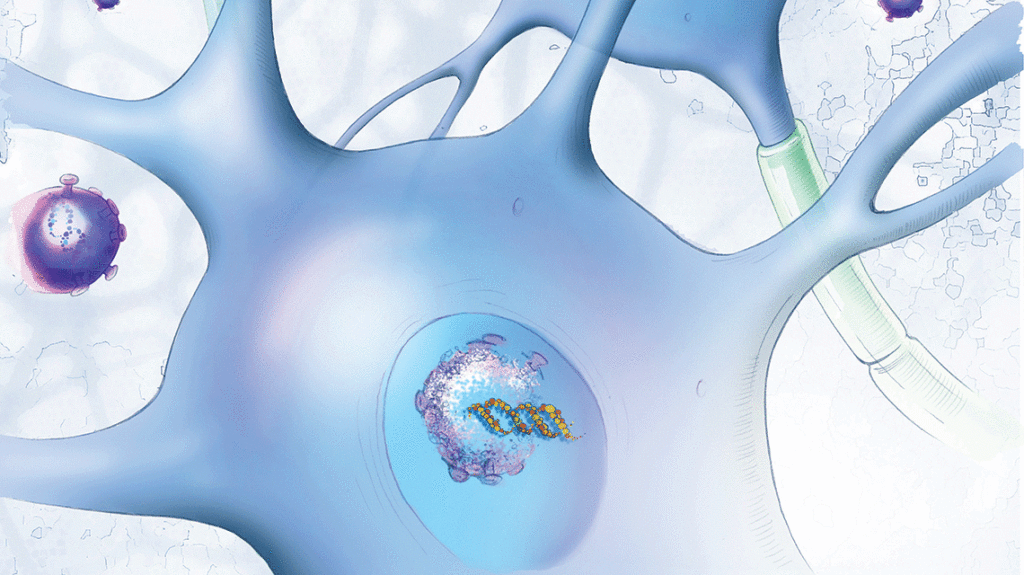
SMA is caused by the absence of the SMN1 gene and subsequent insufficient survival motor protein, which is critical for the maintenance and function of motor neurons. It causes afflicted babies to progressively lose muscle control. Those with the most common form of the disease die within the first two years of life without treatment.
One of three FDA-approved treatments for SMA is a gene replacement therapy called onasemnogene abeparvovec-xioi. The treatment involves a one-time intravenous injection of an adeno-associated virus vector that delivers a fully functional copy of the missing SMN1 gene into motor neuron cells.
Jerry Mendell, MD, a neurologist with Nationwide Children’s Center for Gene Therapy, was pivotally involved in the preclinical work and human trial that demonstrated the safety and efficacy of onasemnogene abeparvovec-xioi. The treatment was approved by the FDA for children diagnosed with any type of SMA under two years of age without end-stage disease. However, the 15 patients treated by Dr. Mendell and his colleagues were all children with type 1 SMA under 8 months of age.
In the new study, Dr. Mendell and colleagues from Nationwide Children’s and three other Ohio children’s hospitals report safety and early outcome data from 21 children (age 1-23 months) treated with onasemnogene abeparvovec-xioi in Ohio.
Megan Waldrop, MD, a pediatric neurologist at Nationwide Children’s and the study’s first author, says their data show the treatment is safe and effective through age 2 years with genotypes predicted to be SMA1, 2 and 3, if proper screening and monitoring is conducted.
“If the children are older and heavier, there is a potential for more liver injury and those patients require closer monitoring and may require longer corticosteroid treatment,” says Dr. Waldrop. “But if they are monitored appropriately, they tolerate the treatment well.”
In addition to close monitoring in the weeks to months post-treatment, Dr. Waldrop and her colleagues emphasize the need for a thorough screening process prior to treatment and social isolation afterwards to minimize the risk of illness.
Overall, the results of the study were promising; all symptomatic patients experienced subjective and objective functional improvements in motor function, while the five children treated prior to symptom onset developed none of the signs of weakness characteristic of SMA.
“We think this is a good treatment for SMA that can make a dramatic clinical impact in the lives of children,” says Dr. Waldrop, who is also assistant professor of Pediatrics and Neurology at The Ohio State University College of Medicine.
“Gene therapy is a remarkable treatment paradigm for certain diseases,” Dr. Waldrop continues. “At the moment, we are able to design gene therapies for some conditions that are recessive, loss-of-function, and involve small genes.
“I suspect that as the field continues to move forward, we will make progress and find ways to broaden the conditions we can treat using gene therapy.”
Reference:
Waldrop MA, Karingada C, Storey MA, Powers B, Iammarino MA, Miller NF, Alfana LN, Noritz G, Rossman I, Ginsburg M, Mosher KA, Broomall E, Goldstein J, Bass N, Lowes LP, Tsao C, Mendell JR, Connolly AM. Gene therapy for spinal muscular atrophy: safety and early outcomes. Pediatrics. 2020;146(3):e20200729.
Image credit: Nationwide Children’s
About the author
Mary a freelance science writer and blogger based in Boston. Her favorite topics include biology, psychology, neuroscience, ecology, and animal behavior. She has a BA in Biology-Psychology with a minor in English from Skidmore College in Saratoga Springs, NY, and a PhD from Brown University, where she researched bat echolocation and bullfrog chorusing.
-
Mary Bates, PhDhttps://pediatricsnationwide.org/author/mary-bates-phd/December 27, 2016
-
Mary Bates, PhDhttps://pediatricsnationwide.org/author/mary-bates-phd/
-
Mary Bates, PhDhttps://pediatricsnationwide.org/author/mary-bates-phd/
-
Mary Bates, PhDhttps://pediatricsnationwide.org/author/mary-bates-phd/
- Post Tags:
- Center for Gene Therapy
- Neurology
- SMA






