An Infant. A Virus. An Emergency IND. A Life Saved.
An Infant. A Virus. An Emergency IND. A Life Saved. https://pediatricsnationwide.org/wp-content/themes/corpus/images/empty/thumbnail.jpg 150 150 Abbie Miller Abbie Miller https://pediatricsnationwide.org/wp-content/uploads/2023/05/051023BT016-Abbie-Crop.jpg- April 30, 2019
- Abbie Miller
Clinician scientists collaborate to use virus-specific T-cells from the mother to successfully treat a systemic adenovirus infection in a preterm infant.
It’s not every day that researchers can say that they’ve written and submitted and emergency investigational new drug (EIND) application to the Food & Drug Administration (FDA) and saved a life.
But that’s what happened when Jeffery Auletta, MD, director of the Host Defense and Immunocompromised Infectious Diseases Program (HDP) in the Division of Infectious Diseases and director of Blood and Marrow Transplant (BMT) Program in the Division of Hematology, Oncology & BMT at Nationwide Children’s Hospital, was consulting on a patient in the Newborn Intensive Care Unit (NICU) with systemic adenovirus infection.
Over the course of just six days last year, Nationwide Children’s clinician scientists conceptualized a treatment that had never been tried on a preterm infant before, received an emergency federal approval for the treatment, and used the therapy to save a life in the NICU.
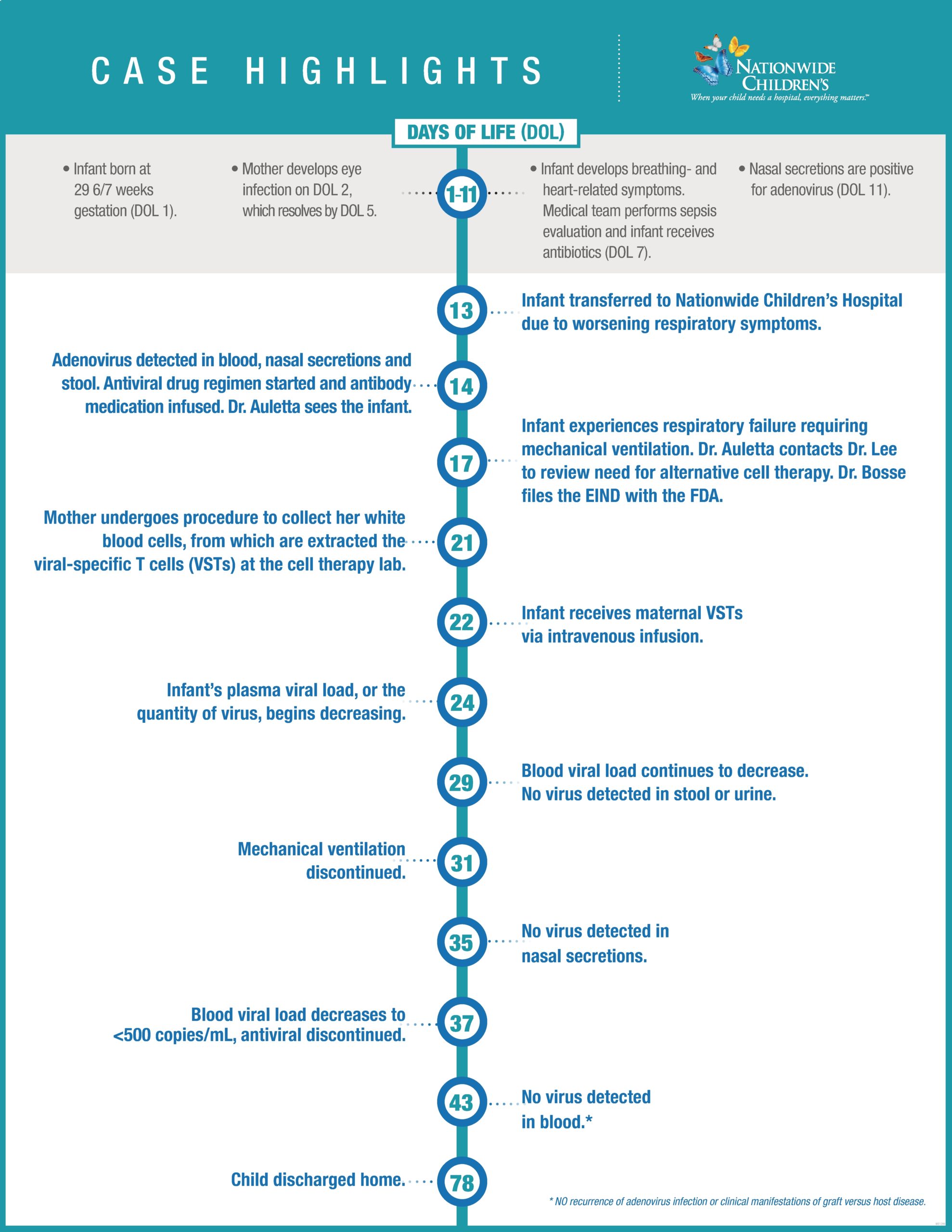
The infant, born at 29 6/7 weeks gestation, was diagnosed with disseminated adenovirus infection at two weeks post-partum after acquiring the virus from the child’s mother. Given the high mortality associated with this infection in a neonate, Dr. Auletta looked outside the box for answers.
“For babies born at term with no other medical considerations, this type of adenovirus infection has a mortality rate around 85%,” says Dr. Auletta, who is also a professor of Pediatrics at The Ohio State University College of Medicine (OSUCOM). “This infant was born preterm and was already struggling with an underdeveloped immune system. The prognosis in this case was not good.”
So, he called Dean Lee, MD, PhD, director of the Cellular Therapy and Cancer Immunology Program at Nationwide Children’s and The Ohio State University Comprehensive Cancer Center (OSUCCC), and together they developed a plan. Dr. Lee’s program uses immune cells to treat disease in an effort to produce better outcomes than outcomes using antiviral drugs, which are not as effective and have toxic side effects.
“Dr. Auletta was working in his infectious diseases capacity, but he knew about what we were doing and what we are capable of. So, he knew what questions to ask,” says Dr. Lee, who is also a professor of Pediatrics at OSUCOM.
They proposed using maternal virus-specific T lymphocytes (VSTs), which are haploidentical, or half matched, to the infant’s immune system. That is, they wanted to take T cells from the mother – who had successfully cleared the infection – and isolate as many of the ones specific to the virus infecting the infant as possible. They would then give the cells to the infant intravenously.
To make it happen, they collaborated with the Cell Therapy Lab in the OSUCCC, which processes cells for BMT and other cell therapies at Nationwide Children’s.
“We had a process for making antiviral T cells that we were just starting to implement in clinical trials on certain viral infections occurring in BMT patients,” Dr. Lee says. “We’d been planning and building to do this work, but we hadn’t thought about the application to the newborn population.”
“For this patient, we wanted to use the same process planned for a cytomegalovirus trial in BMT patients, which had been validated in collaboration by the team in the Cell Therapies Laboratory,” he adds.
Lynn O’Donnell, PhD, director of the Cell Therapy Laboratory at OSUCCC and associate professor of Hematology at OSU COM, led the processing of the maternal cells.
“First, we activated the maternal T cells by exposing them to small fragments of viral proteins,” she says. “Then, we used a specialized machine to tag the activated T cells. Finally, we used those tags to isolate as many activated VSTs and remove as many nonspecific immune cells as possible.”
Because the infant had an immature immune system, Drs. Lee and Auletta suspected that the infant would not reject the T cells. Selecting just the virus-specific T cells would reduce the chance of immune-related complications including graft versus host disease (GvHD).
So the question became, would the FDA agree?
They reached out to Kevin Bosse, PhD, RAC, regulatory operations manager in Drug and Device Development Services to pave the way for the EIND designation.
Most IND applications take months to prepare. But with an all-hands-on-deck approach, the team – 19 researchers, physicians, medical and regulatory staff – pulled everything together that they needed for the EIND in less than 36 hours. The team included experts from Infectious Diseases; Hematology, Oncology, and BMT; Neonatology; Endocrinology; Pathology; Apheresis; and Pharmacy.
“The speed of the decision making and FDA submission was impressive for this process,” says Dr. O’Donnell. “Everyone worked together to make this happen.”
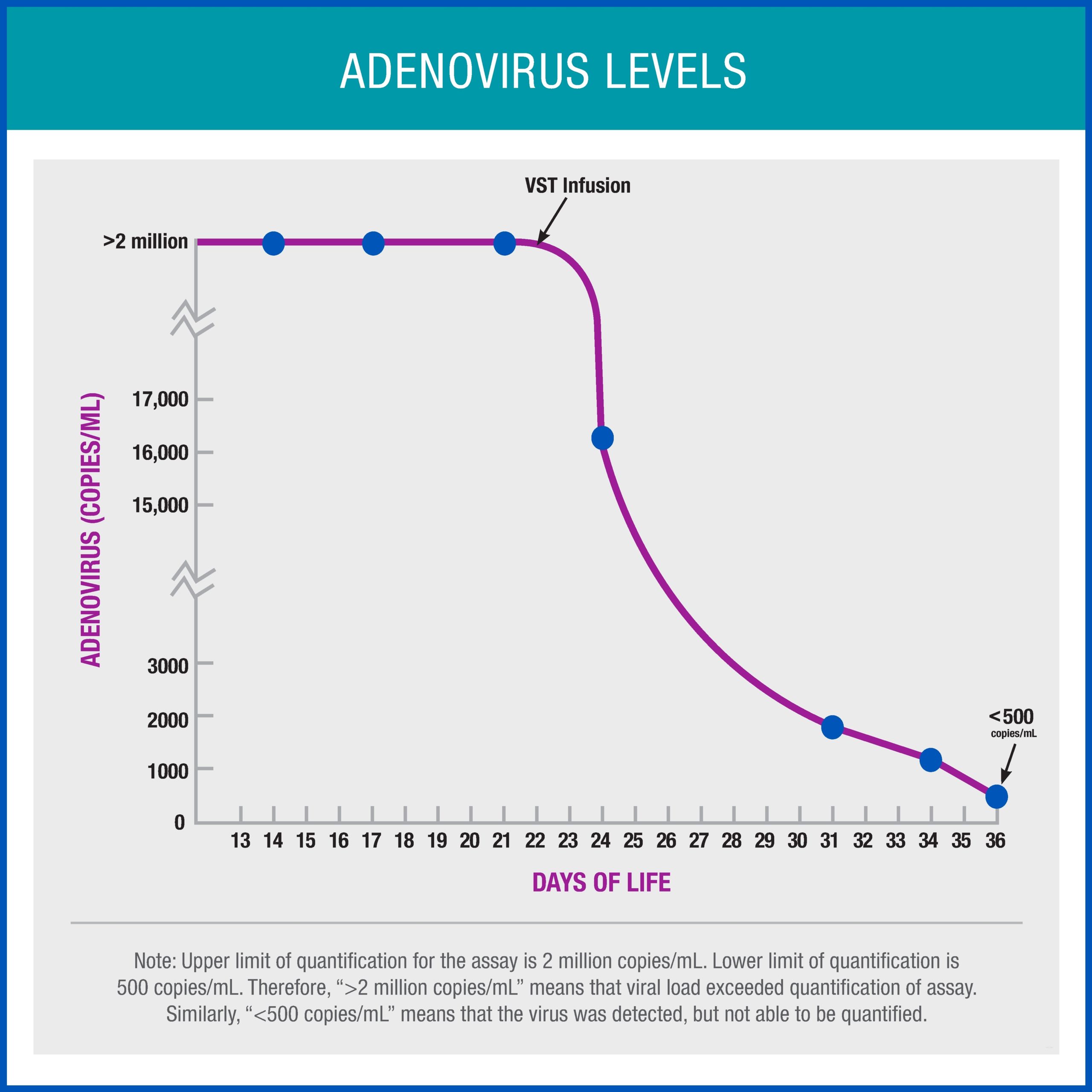
After receiving the novel treatment, the infant’s viral load dropped dramatically and the child’s clinical condition improved, enabling her to ultimately come off life support. Two weeks after receiving VSTs, the infant was free of adenovirus infection and did not develop any complications from the therapy. The infant was ultimately discharged home and has been doing well ever since.
“We say best outcomes for all children. This is us doing it. Doing things that you can’t write a prescription for,” says Dr. Bosse.
“Part of the reason this worked is because we had the infrastructure and staff in place. And we had the dedicated funds to be able to do this,” says Dr. Lee. “This was our first opportunity to pressure test the team we’ve built for cell therapy – we’re proud to say it was a huge success.”
Dr. O’Donnell agrees, “We gave a small amount of VSTs to the infant, and it seemed to be incredibly potent. It will be exciting to see how this type of therapy works in other cases and what other opportunities will come from what we’ve learned in this case.”
Reference:
Auletta JJ, Sánchez PJ, Meyer EK, O’Donnell LC, Cassady KA, Ouellette CP, Hecht S, Diaz A, Pavlek LR, Salamon DP, Gallagher CL, Bradbury H, Welfley SL, Magers J, Armbruster DL, Lamb MG, Nakkula RJ, Bosse K, Lee DA. Adjuvant haploidentical virus-specific T lymphocytes (VSTs) for treatment of disseminated adenovirus infection in a premature infant. The Journal of Allergy and Clinical Immunology. 2019. [Epub ahead of print]
Image credits: Nationwide Children’s
About the author
Abbie (Roth) Miller, MWC, is a passionate communicator of science. As the manager, medical and science content, at Nationwide Children’s Hospital, she shares stories about innovative research and discovery with audiences ranging from parents to preeminent researchers and leaders. Before coming to Nationwide Children’s, Abbie used her communication skills to engage audiences with a wide variety of science topics. She is a Medical Writer Certified®, credentialed by the American Medical Writers Association.
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
- Posted In:
- Features