What’s Next for NEC?
What’s Next for NEC? https://pediatricsnationwide.org/wp-content/uploads/2019/04/Spring-19-Reveal-Final-NEC_header-smaller.jpg 898 504 Abbie Miller Abbie Miller https://pediatricsnationwide.org/wp-content/uploads/2023/05/051023BT016-Abbie-Crop.jpg- April 19, 2019
- Abbie Miller
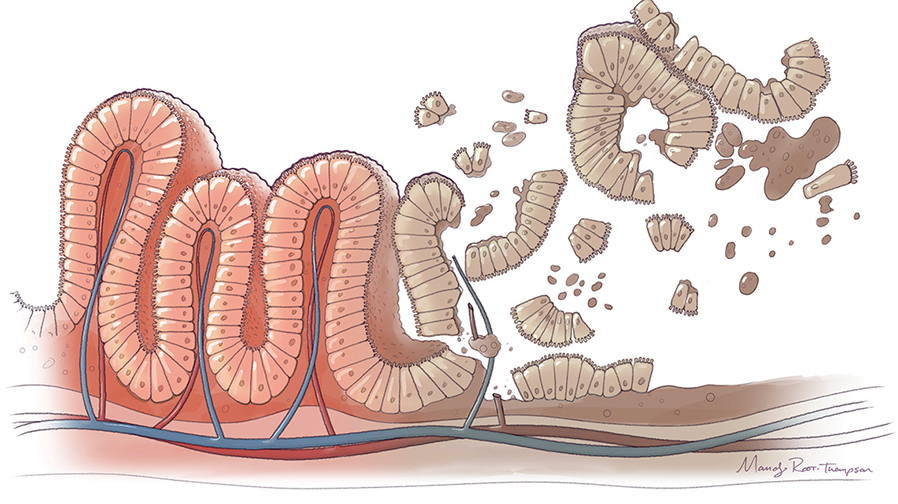
Red. White. Black.
These are the colors of necrotizing enterocolitis, or NEC. When surgeons open the distended abdomens of the tiny infants affected by NEC, they see a mottled mixture of red (inflamed), white (ischemic) and black (dead) tissue. Their first task is to assess whether or not there is enough viable tissue to save. Then, they get to work.
Many things had to happen to get to that moment and those colors. What was the cause? And how can it be prevented?
A DISEASE OF PREMATURITY
The term necrotizing enterocolitis was first used to describe the death of intestinal tissue in premature infants in 1965. In the early 1970s, surgeons developed the surgical protocol to treat the disease.
In 1978, Bell’s criteria, an algorithm developed by Martin Bell, MD, at the Washington University School of Medicine and St. Louis Children’s Hospital, became the uniform clinical staging for infants with NEC. At stage I, NEC is suspected and symptoms are treated medically. At stage II, NEC is proven and medical care continues. In stage III, NEC is advanced: the infant is critically ill with a perforated intestine, and surgery is necessary.
In the past decade or so, advances have focused on preventing the occurrence of NEC, and on developing a deeper understanding of the pathology.
“Despite decades of research, little has changed in terms of prognosis for infants who develop NEC,” says Gail Besner, MD, chief of Pediatric Surgery at Nationwide Children’s Hospital and principal investigator in the Center for Perinatal Research in The Research Institute at Nationwide Children’s. “But the preclinical work that is being reported in the last few years tells us that all of that is about to change. We’re really on the cusp of something big here.”
NEC occurs in 1 to 3 per 1,000 live births, making it a rare disease as classified by the National Institutes of Health. But for clinical professionals in advanced neonatal intensive care units (NICUs) like the one at Nationwide Children’s Hospital – where doctors and nurses care for the smallest and sickest infants – NEC is far too common. More than 90 percent of cases of NEC occur in very low birth weight (<1500 g) infants born at less than 32 weeks gestation.
“It’s on our mind daily,” says Maria Talavera, DO, neonatologist at Nationwide Children’s. “We all struggle with NEC. You’ll see a baby with a little abdominal distention or trouble with a feed, and by the end of your 12-hour shift, the baby could die. Sometimes, it’s that fast. Other times, it lingers, starts, seems to get better, then worsens without notice. For the lucky ones, NEC responds to medical treatment and we never have to operate on them.”
That sudden downward spiral after a fragile infant has survived the first weeks to month of life in the NICU is something that clinicians and scientists want to predict and prevent. But in order to do so, they need to understand what causes NEC.
NEC is characterized by acute and chronic intestinal inflammation. The convergence of many factors, including intestinal immaturity, the microbiome, the immune response and pathogen exposure, can ultimately lead to systemic sepsis and multi-system organ failure in patients with NEC.
Risk factors for the development of NEC in premature infants include hypoxia, formula feeding, abnormal bacterial colonization of the bowel, increased release of inflammatory mediators, intestinal ischemia-reperfusion injury, use of acid-reducing medications and transfusion-associated gut injury (TRAGI).
“In premature infants, you have sort of a perfect storm of factors that can lead to NEC,” says Dr. Talavera. “Mucosal injury and bacterial overgrowth compromise the immature intestinal epithelium, while the abnormal flora takes advantage of the immature immune system.”
According to Dr. Besner, finding the answer to NEC has been challenging.
“One challenge regarding NEC is the inability to identify exactly which babies are most likely to get the disease. If we could predict this with certainty, then we would know exactly which babies to treat with potential novel therapeutics for the disease,” she explains. “As our understanding of NEC pathology increases and as biomarkers predictive of NEC are identified, novel strategies to treat the disease can be employed.”
HARNESSING THE POWER OF BREAST MILK
Most premature babies are initially placed on total parenteral nutrition (TPN) intravenously until their immature intestines are capable of tolerating feeds.
Giving breast milk orally is the first step in preventing NEC, according to Dr. Talavera.
“Just a drop of breast milk – preferably mom’s but possibly from a donor – can start the process of coating the digestive tract with all the good things that will help the baby thrive,” she says. “We can even just paint the mouth with a little breast milk from day zero. Getting more oral feeds as early as possible is an important goal for preventing NEC.”
So what is it about breast milk that is so protective? This is an area of research currently being explored by clinician-scientists around the world. It is likely that many components of breast milk play a role in modulating the microbiome, immune system and gut development to prevent NEC.
One such component includes exosomes, nano-sized vesicles that are secreted by all cell types including stem cells.

“We have shown that several different types of stem cells can protect the intestines from NEC in animals,” says Dr. Besner. “Furthermore, exosomes secreted by these stem cells provide the same benefit as the stem cells themselves without the potential risks associated with stem cells, such as tumor growth. And, exosomes are present in human breast milk.”
In preclinical studies, Dr. Besner’s team has purified breast milk-derived exosomes and fortified breast milk feeds with them. The exosome-fortified breast milk reduced the incidence of NEC in animal models.
“Exosomes from donor milk are easily purified and could be used to fortify the feeds for premature infants at risk for NEC,” she says.
“Breastfeeding prevents NEC, but even with best nursing practices, NEC persists,” Dr. Besner adds. “You can do everything right, and NEC can still develop. We need advanced strategies for prevention and treatment if we are to eliminate NEC from the NICU.”
PROBIOTICS – GOOD FOR THE GUT
Researchers have long been investigating the possibility that probiotic therapy could prevent NEC. And some probiotic protocols are in place in some NICUs across the country. However, none of them have produced the compelling results that researchers hope for.
One of the problems with traditional approaches to probiotic therapy is the delivery system. For probiotics to work, they must survive the acidity of the stomach and the host immune system, and then take root in the gut, where they are expected to compete with pathogenic bacteria and flourish. All probiotic clinical studies to date have used free-living (planktonic) probiotic formulations, which do not optimally display these required characteristics.
“The practice of giving high doses of probiotics daily or even with every feed has its risks,” says Dr. Besner. “One of those risks is bacteremia, or bloodstream infection, from the probiotic administered. It is desirable to increase the efficacy of the probiotic in order to decrease the amount needed while still maintaining protective effects.”
Dr. Besner and her colleagues, including Steve Goodman, PhD, Michael Bailey, PhD, and Lauren Bakaletz, PhD, recently licensed a technology that solves this problem – administering the probiotic in a biofilm state provides near elimination of NEC with one dose in preclinical studies.
Their probiotic of choice, Lactobacillus reuteri, is incubated with maltose-filled dextranomer microspheres (Lr-DM-Malt). In one study published in the Journal of the American College of Surgeons in 2017, the administration of a single dose of Lr-DM-Malt reduced the incidence of NEC from 70 percent to 21 percent in the animal model. Administration of Lr without the microspheres, that is, without being in biofilm state, did not protect against the development of NEC. In a follow-up study published in American Journal of Physiology Gastrointestinal and Liver Physiology, the investigators filled the microspheres with sugars that promoted increased biofilm formation, making the probiotic formulation even more effective.

“We know that bacteria are stronger and more resistant in the biofilm state,” says Dr. Goodman. “They are in their optimal physiologic state in a biofilm, and this gives them a better chance to affect the digestive tract.”
Dr. Goodman uses the following analogy to explain. Imagine that you have two runners who are getting ready for a race. One has just rolled out of bed and might not have had breakfast. This one is like the planktonic probiotics. The other has been fed, warmed up, and is in peak physiologic state. This is probiotics in the biofilm state. Which do you think will be the most effective?
Another aspect of the Lr-DM-malt microsphere technology that excites the researchers is the GRAS designation of each of the components. GRAS is a Food and Drug Administration category that stands for “Generally Recognized as Safe.”
“We’re hopeful that the GRAS designation of each of the components will be helpful in getting the technology into the clinic,” says Dr. Besner. “These are all well-known components combined in a new way to produce what we believe will be an extraordinary outcome.”
THERAPEUTICS BASED ON TLR SIGNALING
David Hackam, MD, PhD, surgeon-in-chief and principal investigator at Johns Hopkins Children’s Center, and his team are adding their own approaches to the arsenal against NEC. In the past several years, they have published a series of papers that demonstrate the role of toll-like receptors in the pathology of NEC, specifically, the role of TLR-4.
TLRs are part of the innate immune system and play a key role in identifying pathogens. But that’s after birth. In utero, TLRs – specifically TLR4, which has been associated with NEC – play a different role.
“TLR4 expression in the fetal gut helps the gut develop properly,” explains Dr. Hackam. “Typically, that high level of expression is turned off before birth.”
Dr. Hackam and his team have shown experimentally that high levels of TLR4 expression in animal models lead to NEC. They’ve supported the bench work with evidence from the NICU. Levels of TLR4 in the intestines of infants with NEC are also high. Conversely, babies without NEC have low levels of TLR4.
NEC occurs after bacteria enter the gut. Recall the onset is typically a week to months after premature delivery. In the presence of bacteria, the TLR4 takes on an inflammatory role, prompting a cytokine storm that ultimately causes inflammation and restricted blood flow to the gut – in other words, NEC.
“The good news is that TLR4 is ultimately targetable,” says Dr. Hackam. “We know that some things help to regulate TLR4. Specifically, amniotic fluid and breast milk.”
These substances serve as inspiration for his team as they are working to develop several drugs that prevent TLR4 activation.
Another avenue they are exploring is the TLR9 pathway. “In NEC, you have too much expression of TLR4 and not enough TLR9,” says Dr. Hackam. “By increasing TLR9, we could balance the ratio of TLR4 to TLR9 in an effort to prevent NEC.”
BEYOND SURVIVAL
NEC carries a 30 to 50 percent mortality rate. And for those patients that survive, the downstream effects can be lifelong. Depending on how much of the intestine is surgically removed, the infant may require G-tube feeding for the short or long term. Short gut syndrome, also called intestinal failure, requires long-term follow up with gastroenterologists. For some survivors of NEC, getting proper nutrition will be a lifelong challenge.
Nearly 50 percent of survivors of NEC develop significant developmental and cognitive disability.
“The neurodevelopmental injury that results from NEC is both more severe and more difficult to treat than that caused by prematurity in general,” says Dr. Hackam. “Our program is also investigating the pathways that cause the neurodevelopmental problem.”
The best way to prevent NEC-associated neurodevelopmental problems is to prevent NEC. But in the cases where NEC is present, a therapeutic approach to minimize or eliminate the damage to the brain would be life altering.
In an article published last year in Science Translational Medicine, Dr. Hackam and his team show that TLR4 expression could be a culprit in the brain injury in addition to the intestinal injury. Using their animal model of NEC, they showed that TLR4 activation induced microglial activation in the brain. The study also tested an intervention – a dendrimer-based nanotherapeutic approach – that targeted the activated microglia to prevent NEC-associated neurologic dysfunction in the model.
In an effort to help mitigate the effects of short bowel syndrome, Drs. Hackam and Besner each have research programs dedicated to developing tissue -engineered intestinal grafts. As with other tissue-engineered organs, the idea involves using a biodegradable scaffold seeded with stem cells that will ultimately develop into a living, growing, functional intestine.
“Tissue engineering is a quickly growing field. We’re excited about the prospect of being able to help children who have lost a significant portion of their intestine,” says Dr. Besner. “We are still in early preclinical work and look forward to further developing this program.”
A BRIGHT FUTURE
“NEC is scary for parents, it’s scary for physicians,” says Dr. Talavera. “But we’ve shown that little changes can improve outcomes.”
For example, a three-step quality improvement protocol implemented in the Nationwide Children’s NICUs reduced the incidence of NEC from 8 percent to only 3.1 percent among very low birthweight babies.
“The three strategies we employ from a QI perspective include early human milk feedings, no feedings during blood product transfusions and restricting the use of acid suppressive medication, such as ranitidine,” says Dr. Talavera, who was the lead author on the publication in Pediatrics that reported the findings of the protocol.
Access to human donor milk is an important part of the equation.
“In Ohio, we’re fortunate to have a milk bank resource,” says Dr. Talavera. “Mothers of NICU infants need additional support to pump and maintain a supply. The importance of breastfeeding and providing breast milk is engrained in the NICU, but still, it may not be an option for every mother. That’s why donor milk is so important.”
Through QI, therapeutic development and a deepening understanding of how NEC forms, the future is looking bright for infants who are at risk for NEC.
“For many years, the treatment of babies with NEC has been plagued with many unknowns that add to the pain and suffering caused by this disease,” says Dr. Hackam. “Now, we’re finally able to offer hope. All of the advances in the last several years were inconceivable before.”
Dr. Besner agrees, “With the advances that are in the pipeline, I think we can expect big changes in outcomes. It is our hope that in another few years, NEC could be a relic of the past. That’s what we work toward.”
This story was part of the Spring/Summer 2019 print issue. Download the full issue.
References:
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based on clinical staging. Annals of Surgery. 1978 Jan;187(1):1-7.
- Hackam DJ, Afrazi A, Good M, Sodhi C. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clinical and Developmental Immunology. 2013 May;2013:475415.
- Nino DF, Zhou Q, Yamaguchi Y, Martin LY, Wang S, Fulton WB, Jia H, Lu P, Prindle Jr T, Zhang F, Crawford J, Hou Z, Mori S, Chen LL, Guajardo A, Fatemi A, Plentikov M, Kannan RM, Kannan S, Sodhi CP, Hackam DJ. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in the mouse brain. Science Translational Medicine. 2018 Dec 12;10(471).
- Olson JK, McCulloh CJ, Navarro JB, Mashburn-Warren L, Goodman SD, Besner GE. A new role for an old adversary: Biofilms for protection against experimental necrotizing enterocolitis. Journal of the American College of Surgeons. 2017; 255(4);S147.
- Olson J, Navarro J, Allen J, McCulloh C, Mashburn-Warren L, Wang Y, Varaljay V, Bailey M, Goodman S, Besner G. An enhanced Lactobacillus reuteri biofilm formulation that increases protection against experimental necrotizing enterocolitis. American Journal of Physiology — Gastrointestinal and Liver Physiology. 2018 May 31;315(3):G408-G419. [Epub ahead of print]
- Talavera MM, Bixler G, Cozzi C, Dail J, Miller RR, McClead R Jr, Reber K. Quality improvement initiative to reduce the necrotizing enterocolitis rate in premature infants. Pediatrics. 2016 May;137(5).
Image credits: Nationwide Children’s
About the author
Abbie (Roth) Miller, MWC, is a passionate communicator of science. As the manager, medical and science content, at Nationwide Children’s Hospital, she shares stories about innovative research and discovery with audiences ranging from parents to preeminent researchers and leaders. Before coming to Nationwide Children’s, Abbie used her communication skills to engage audiences with a wide variety of science topics. She is a Medical Writer Certified®, credentialed by the American Medical Writers Association.
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
- Posted In:
- Features







