Challenges in Clinical Decision-Making for Patients with Spinal Muscular Atrophy
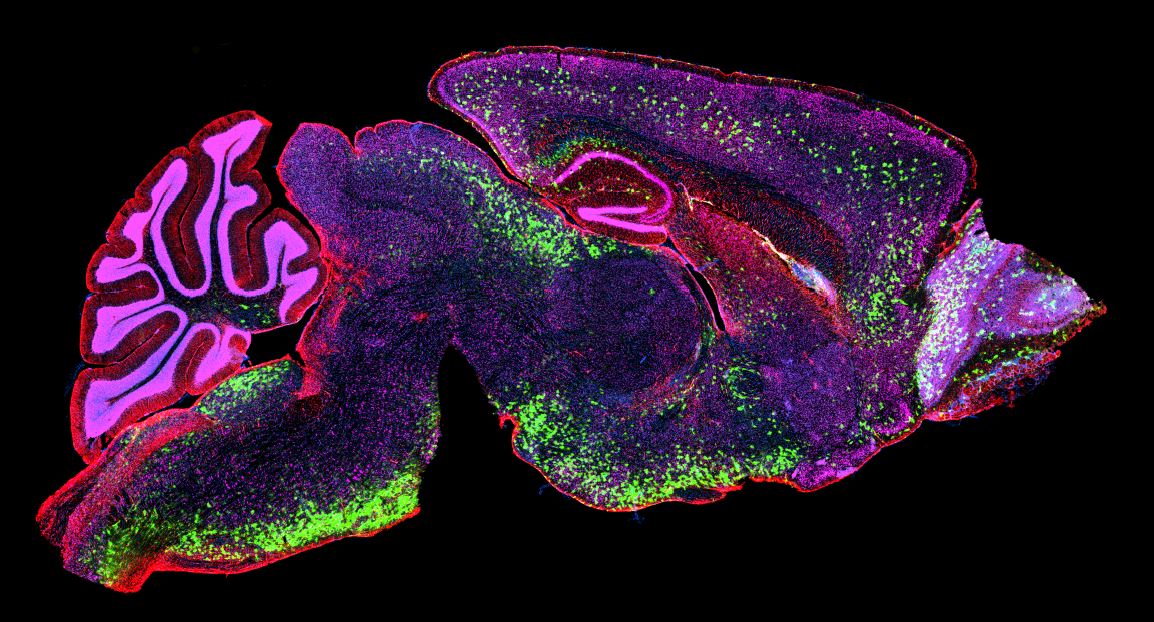
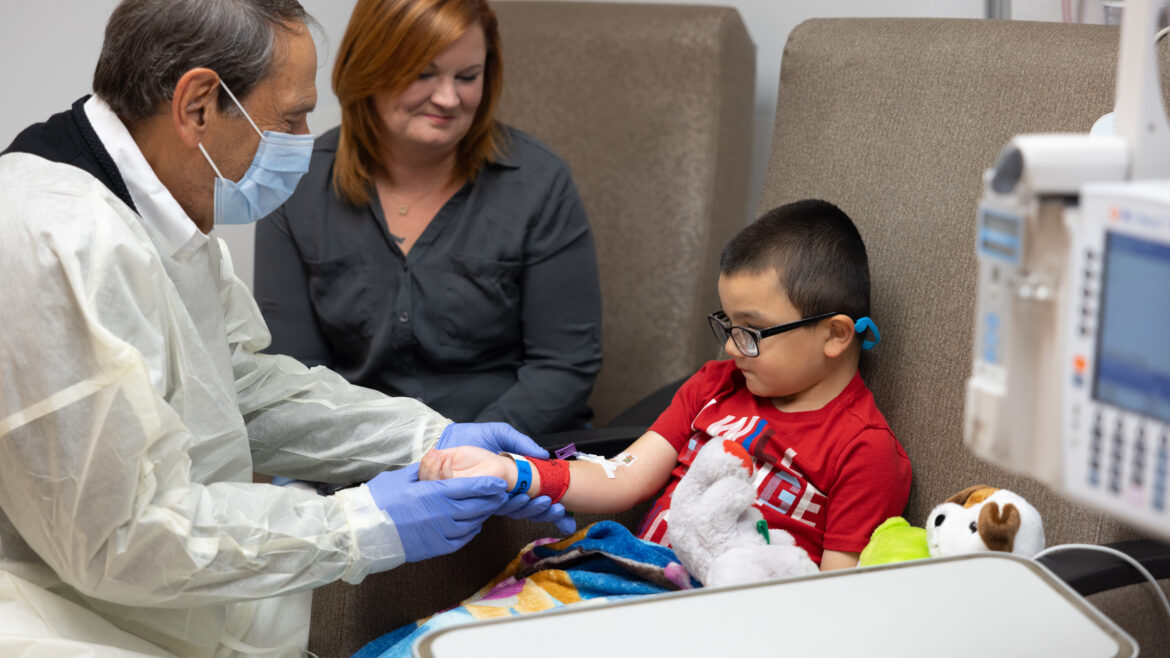
Challenges in Clinical Decision-Making for Patients with Spinal Muscular Atrophy https://pediatricsnationwide.org/wp-content/uploads/2024/12/091619ds3489-1024x683.jpg 1024 683 Mary Bates, PhD https://secure.gravatar.com/avatar/c6233ca2b7754ab7c4c820e14eb518c8?s=96&d=mm&r=gThere are three approved genetic therapies for SMA, but little guidance in treatment selection. Spinal muscular atrophy (SMA) was once a leading cause of inherited infant death in the United States. Today, there are three genetic therapies approved by the Food and Drug Administration to treat SMA — but no clinical trials directly comparing treatment…