Biofilms: The Good, the Bad & the Groundbreaking
Biofilms: The Good, the Bad & the Groundbreaking https://pediatricsnationwide.org/wp-content/uploads/2024/09/eDNA-matrix-with-immunolabeled-DNABII-proteins-081424_NEW-1024x615.jpg 1024 615 Katie Brind'Amour, PhD, MS, CHES Katie Brind'Amour, PhD, MS, CHES https://pediatricsnationwide.org/wp-content/uploads/2021/03/Katie-B-portrait.gif- September 23, 2024
- Katie Brind'Amour, PhD, MS, CHES
Decades of research into the structure and function of bacterial biofilms have begun to pay off in the form of imminent clinical applications capable of harnessing both the protective and problematic aspects of this universal phenomenon.
Imagine a hospital emergency department filled with patients — those with painful ear infections, recurrent urinary tract infections, fevers and swelling after surgery, signs of infection at the site of a wound. Then picture the hospital’s inpatients — a child with cystic fibrosis, an elderly patient with pneumonia, a patient with a drug-resistant skin rash.
Now imagine all of these patients have essentially the same problem.
And the solution is on the horizon.
A Biofilm Primer
Nearly 5 million people die every year due to bacterial infections that resist antimicrobial therapies — accounting for almost 1 in every 10 deaths worldwide. Approximately 70% of these deaths are attributable to just seven pathogens, which together go by the moniker ESKAPEE: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp. and Escherichia coli.
ESKAPEE pathogens, at the root of many of the familiar infections affecting the patients described above, often “escape” traditional pathogen-specific antibiotics or even high-dose, broad spectrum antimicrobial therapies. They linger on surfaces and resist both our immune system’s defenses and aggressive medical therapies, either becoming recalcitrant, life-threatening infections or subsiding only to recur over time.
The reason? Biofilms.
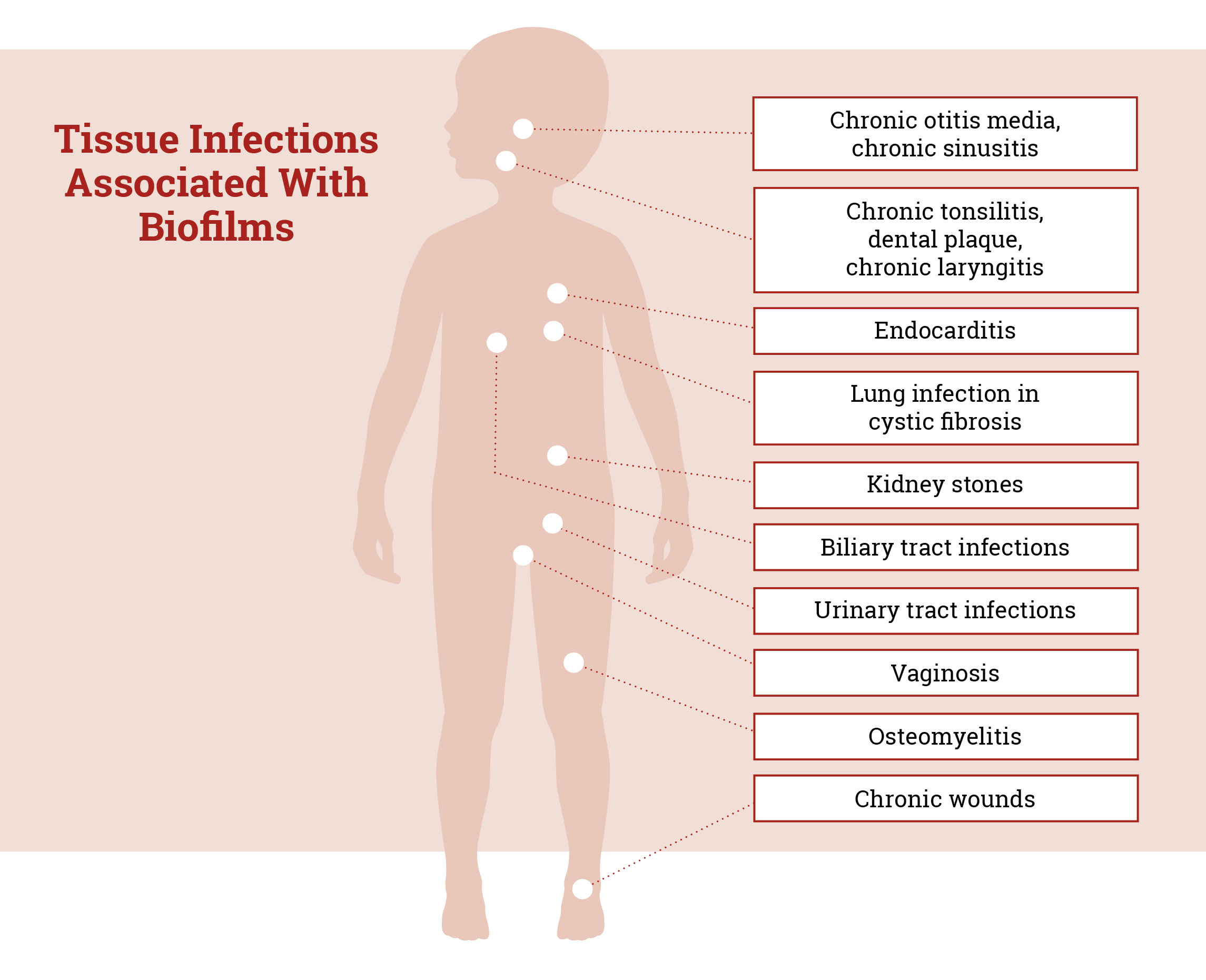
About 80% of bacterial infections in humans involve biofilms — naturally formed communities of bacteria that construct a web-like matrix inside the body using extracellular DNA (eDNA) from both the host and the bacteria. The resultant film physically shields the bacteria from immune cells, medications, adverse environments and even DNA-destroying enzymes.
The protected bacteria become more than 1,000 times as resistant to antibiotics as their free-floating counterparts in the blood. Protected by the biofilm, they can thrive without wasting energy on additional defense mechanisms or extensive cell division.
Over the past few decades, researchers at Nationwide Children’s Hospital have chipped away at understanding the various biochemical and structural elements of these biofilms.
For example, they discovered that biofilms form when eDNA is wrapped into a rare, enzyme-resistant configuration known as Z-DNA.
More importantly, however, they found the linchpin protein for virtually every biofilm: DNABII. This protein family holds the matrix together — making it a prime target to literally make biofilm walls come tumbling down.
We have identified what we believe is the Achilles heel common for all biofilms, regardless of which organisms produce them. By targeting that Achilles heel, we can rapidly force the bacteria out of the biofilm.”
— Lauren Bakaletz, PhD

Targeting the Underlying Threat
“We have identified what we believe is the Achilles heel common for all biofilms, regardless of which organisms produce them,” says Lauren Bakaletz, PhD, director of the Center for Microbial Pathogenesis at the Abigail Wexner Research Institute (AWRI) at Nationwide Children’s and one of the two leading biofilm experts who helped reveal the role of DNABII.
Dr. Bakaletz and colleagues designed a humanized monoclonal antibody, called HuTipMab, that recognizes and binds to the critical DNABII proteins. As DNABII becomes increasingly unavailable to the biofilm, this DNA-dependent shield quickly weakens and collapses.
“By targeting that Achilles heel, we can rapidly force the bacteria out of the biofilm,” Dr. Bakaletz explains. “There is a period of time — a really important, opportunistic period of time — where they have what we call the newly released or NRel phenotype, when they are incredibly sensitive to antibiotics and our immune system.”
Dr. Bakaletz’s long-term research colleague and fellow discoverer of biofilm’s DNABII-dependence, Steven Goodman, PhD, principal investigator in the Center for Microbial Pathogenesis at AWRI, likens the NRel phenotype to the sluggish, discombobulated state most people would be in upon a midnight wake-up call followed by a push out the door to run a marathon. This period of shock lasts about 6 to 12 hours, during which the NRel bacteria are nearly defenseless.
This is another potential win for patients and antimicrobial stewardship, as much smaller doses of antibiotics may be required, and — when coupled with the immune system — should result in total eradication of these newly released pathogens.
Even better, DNABII proteins are pathogen-agnostic, meaning HuTipMab works regardless of the primary bacteria at play. HuTipMab even disrupts biofilms formed by the notorious ESKAPEE pathogens, making them extremely susceptible to even small doses of common antibiotics. These findings, published in Frontiers in Microbiology, represent exciting progress toward a possible solution to the global antimicrobial resistance crisis.
The real test, of course, comes in the form of in vivo studies and clinical trials, and so far, all signs point to “go.”
Paradigm-Shifting Clinical Promise
“Lauren’s preclinical models are spectacularly accurate when it comes to results that are applicable to human beings, which is hard to find,” says Dr. Goodman. Distinct models of human infection — middle ear, necrotizing enterocolitis, periodontal disease and lung infections — complement dozens of in vitro and biospecimen studies for biofilm-associated problems.
To facilitate the advancement of HuTipMab, Drs. Bakaletz and Goodman became the scientific co-founders of a company called Clarametyx Biosciences and licensed the technology — now called CMTX-101 — to them to accelerate its development.
“CMTX-101 represents a potentially transformative therapy to combat difficult-to-treat bacterial infections,” says Chuck McOsker, PhD, co-founder and chief scientific officer for Clarametyx. “Overall, we are hopeful that our therapeutic antibody can reduce the frequency, severity and duration of bacterial infections, resulting in a significant improvement in the quality of life for patients and their families.”
Clarametyx has already garnered $33 million in Series A funding to bankroll its first initiatives: a Phase 1b/2a clinical trial for the use of CMTX-101 in people with cystic fibrosis and a Phase 1 trial using CMTX-101 as an adjunct to standard-of-care antibiotics for bacterial pneumonia.
The early-phase trials should allow assessment of the antibody’s safety and tolerability and provide data on opportunities to enhance timing, dosage, delivery method and future study designs.
“Developing an effective anti-biofilm treatment could dramatically increase the effectiveness of current antibiotics,” says Dr. McOsker. “We see a tremendous opportunity for anti-biofilm therapies like CMTX-101 to deliver improved clinical outcomes for a wide range of additional biofilm-associated bacterial infections, including acute and chronic wounds, prosthetic joint infections [PJI] and osteomyelitis, bacterial exacerbations in chronic obstructive pulmonary disease and non-cystic fibrosis bronchiectasis, among others.”
John Hamilton, MD, PhD, instructor and physician-scientist in the Department of Orthopedic Surgery at Rush University Medical Center, agrees.
“Use of anti-DNABII antibody could fill a much-needed clinical gap for preventing and treating PJI through directly targeting bacterial biofilm,” says Dr. Hamilton, who reached out to Drs. Bakaletz and Goodman when he was searching for new ways to fight PJI.
Despite improvements in safety over the past couple of decades, PJIs still affect tens of thousands of patients per year in the U.S. alone and can result in life-altering sequelae, such as long periods of high-dose antibiotics, reoperation and, in severe cases, amputation. The two groups collaborated to test the anti-DNABII antibody in animal PJI studies via intravenous injection, intraarticular injection and even an anti-DNABII antibody coating directly on the orthopedic implants.
“We felt their technology was the perfect fit to test in the setting of PJI,” says Dr. Hamilton. “It could substantially enhance PJI treatment success and reduce mortality, which can be as high as 25% over 5 years. It could also be added to the antibiotic and surgical treatments used currently for PJI, and perhaps even eliminate the need for surgical treatments for PJI altogether.”
Preliminary data from the animal studies suggests the anti-DNABII antibody may effectively reduce the risk of PJIs, and Dr. Hamilton has secured funding from the National Institutes of Health (NIH) to further study these approaches.
Use of anti-DNABII antibody could fill a much-needed clinical gap for preventing and treating PJI through directly targeting bacterial biofilm.”
— John Hamilton, MD, PhD

Work To Be Done
Questions that biofilm researchers still have to answer include whether therapies could have detrimental off-target effects, how often potential patients would have to be dosed, how long anti-biofilm antibodies stay in the body, and what methods of delivery are appropriate for each application.
At Nationwide Children’s, early research has already confirmed that anti-DNABII antibodies administered into the middle ear via tympanostomy tubes cause less disruption to both the nasopharyngeal and gut microbiome than standard-of-care antibiotics in multiple preclinical models.
This initial work represents an important advantage compared to gold-standard treatments and is a major focus of Michael Bailey, PhD, principal investigator in the Center for Microbial Pathogenesis at AWRI. He specializes in characterizing the microbiome and in studying possible mechanisms at play in the gut-brain connection.
“There is increasing evidence that the natural microbiota, primarily in the gut but in other areas in the body as well, contribute to overall health, and growing awareness that every time we use a medication, there is potential to disrupt the body’s microbiomes,” says Dr. Bailey, who explains that this is particularly true for antibiotics.
This opened up a new area for consideration: what about good bacteria? Avoiding disruption of positive microbiota is critical to both patient wellbeing and long-term utility of the antibody as a solution to the problem of antimicrobial resistance.
“With our studies on anti-biofilm treatments targeted at the middle ear, they look like they’re leaving the body’s microbiomes intact — maintaining healthy microbiomes — and not inducing dysbiosis,” says Dr. Bailey of the team’s work in animal models.
The multidisciplinary research team started thinking about big-picture implications of managing biofilms and the microbiome, and struck another golden idea.

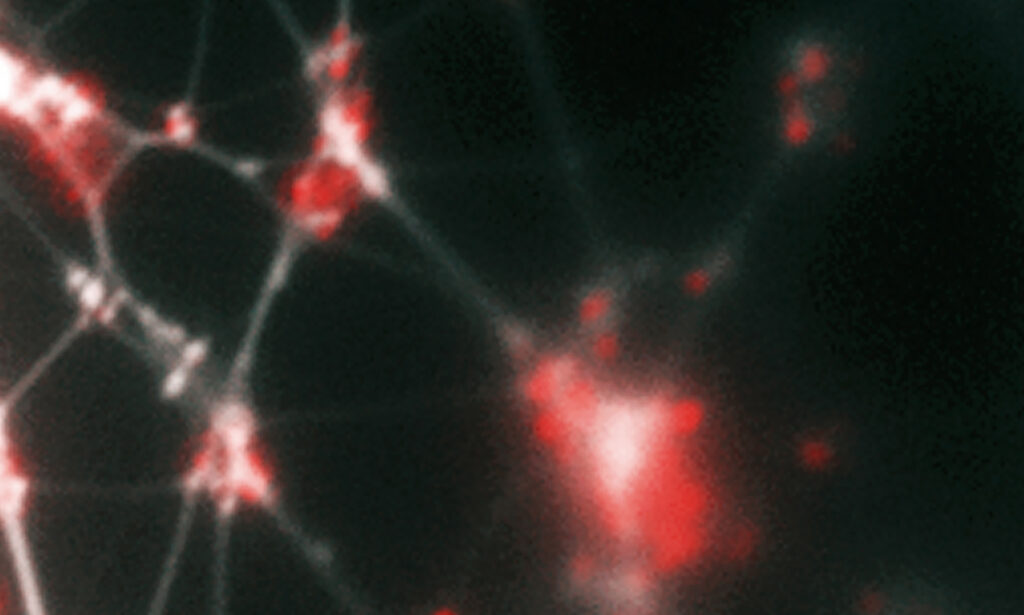
This figure shows a biofilm formed in vitro by two human respiratory pathogens (nontypeable Haemophilus influenzae and Moraxella catarrhalis). NTHI are rod-shaped, while M. catarrhalis are the spheres. The NTHI has never been known to completely cover the M. catarrhalis when they grow together. Drs. Bakaletz and Goodman posit this provides protection for both bacterial species due to the unique properties of each. The more open areas are likely water channels that feed/bathe the biofilm, and the stringy fibers are the extracellular DNA that a portion of the bacterial put out into the extracellular environment to build the supportive structure of the biofilm matrix.
Promoting the Good
More than 10 years ago, Gail Besner, MD, chief of pediatric surgery at Nationwide Children’s, gave a cross-disciplinary talk to the gastroenterology faculty about her research in necrotizing enterocolitis (NEC) and protecting the neonatal intestines from injury. After her presentation, then-president of AWRI at Nationwide Children’s, John Barnard, MD, told her she needed to connect with Drs. Goodman and Bailey right away.
“When you talk to physicians and surgeons about biofilms, what immediately comes to mind is that biofilms are our enemy, because when produced by pathogenic bacteria they prevent treatment of infection,” says Dr. Besner, who admits she was initially skeptical of using biofilms to treat her most vulnerable neonatal patients, but was willing to explore just about anything that could help. “I think I speak for every pediatric surgeon when I say that we would love to never have to operate for NEC in these babies again.”
NEC is a problem of gut dysbiosis in premature babies, with a mortality rate as high as 50%. Severe forms can necessitate removal of large portions of the intestines, and it often causes long-term developmental delays. A lack of bacterial diversity in the gut, along with disproportionately high levels of harmful Gram-negative species, contributes to development of the disease.
For many years, neonatal intensive care units (NICUs) around the world prophylactically treated babies with daily doses of probiotics — often with Limosilactobacillus reuteri, a healthy bacterial species isolated from breastmilk that competes with harmful bacteria to restore and maintain balance of intestinal microbes.
Unfortunately, efficacy of this intervention has been mixed, and in September of 2023 the Food and Drug Administration issued a warning against the off-label use of probiotics for preterm infants, since the preparations used to date have not been tested for purity and are not FDA-approved.
A primary problem with traditionally administered probiotics is their durability — they often don’t survive past the acidic environment of the stomach to reach their target in the intestines. Those that do make it to the gut lack staying power.
But biofilms are built to last.
Drs. Goodman and Bailey had started exploring the possibility of using biofilms to help beneficial probiotics actually reach the small and large bowel — and Dr. Barnard believed that Dr. Besner’s fragile patients represented a group in great need of such an advancement.
Fast-forward several years and the team has published numerous studies in multiple animal models supporting the approach, confirming that just a single dose of L. reuteri administered in its biofilm state using biocompatible microspheres can dramatically reduce the incidence of NEC.

Limosilactobacillus reuteri forms a biofilm on maltose-filled dextranomer microspheres. When administered as a single dose to an animal model, Lr-DM-Malt reduced the incidence of NEC dramatically.
“We can induce beneficial L. reuteri to form a biofilm, which makes the bacteria more acid-resistant when ingested and more capable of binding to intestinal epithelial cells once they’re past the stomach,” says Dr. Besner of the unique probiotic technology. “Then they can actually exert their beneficial effects, because they’re more able to combat the immune system of the host, they’re more resistant to antibiotics and they’re more capable of competing with the pathogenic bacteria around them.”
The trio, together with Dr. Bakaletz, has since patented and licensed the technology to a start-up company called Scioto Biosciences.
As Scioto Biosciences and the research team of co-founders and scientific consultants examined options for their first human trial for the probiotic biofilm microspheres, they landed on a rather unexpected condition: autism.
“It has been shown in the literature that L. reuteri stimulates a hormone called oxytocin, which is out of balance in many people with autism spectrum disorder and may contribute to social behavioral deficits,” explains Dr. Bailey.
The blinded, placebo-controlled Phase 1 safety trial revealed that not only were the L. reuteri microspheres safe and tolerable in a small sample of otherwise healthy adults with autism, but that there is also preliminary evidence for improvement in behavior during periods of daily supplementation.
“The only drugs approved for autism so far are high-powered antipsychotics, and here we have something with the potential for an industry-leading safety profile — we say it’s like eating a protein bar,” says Joe Trebley, PhD, co-founder and chief executive officer at Scioto Biosciences. The company plans to launch a larger Phase 2 autism trial in 2025. “The formulation seems incredibly promising, not just in a safety and GI sense, but also for modulating social behaviors.”
NEC models from the labs at Nationwide Children’s also demonstrated improved neurodevelopment and a decrease in brain inflammation, suggesting the probiotic biofilm has both GI- and neuro-protective effects.
As the team continues to collect sufficient data to justify an early-phase trial in premature infants with NEC, opportunities for alternative probiotic applications abound, from gastrointestinal disorders such as irritable bowel syndrome and Clostridioides difficile infections to psychiatric and immunologic conditions linked to disruptions in gut-brain balance.
More Contenders in the Race to the Clinic
The research team has not stopped at just two approaches to biofilm manipulation.
Dr. Bakaletz’s original foray into biofilm research involved the development of a vaccine against middle ear infections. This approach uses an anti-DNABII vaccination to teach the body to prevent the formation of pathogenic biofilms in the middle ear and other sites of infection in high-risk populations, such as children with chronic or recurrent ear infections.
The technology, licensed to Clarametyx for development, is also being studied for use in pulmonary diseases such as cystic fibrosis. Preclinical data show potential for reduced antibiotic use in these populations, as well as prevention of the devastating consequences of recalcitrant infections.
In addition, the team continues to study biofilms and the way the host identifies and responds to them.
“Something we discovered that I think will have real legs is that our immune systems actually do not try to get rid of biofilms — not because they don’t want to, but because they’re ‘afraid’ to,” says Dr. Goodman. “The goal is to prevent them from proliferating, because the host has no mechanism to know how many bacteria are in the biofilm; it could be 10, or it could be 10 billion, so the immune system would rather cordon it off.”
This is what happens with many infections, such as recurrent sinusitis or even tuberculosis. The immune system encapsulates the biofilm, allowing it to essentially go dormant for long periods of time. When it rears its head, treatments quiet it down again until the process repeats.
Drs. Goodman, Bakaletz and their laboratory teams discovered a protein in the innate immune system called HMGB1 that, much like antibodies to bacterial DNABII proteins, can quickly disrupt a biofilm to release the bacteria. HMGB1 typically triggers an inflammatory response, but when engineered with a single amino acid change, it keeps inflammation in check while disrupting the biofilm’s structure.
Separately, DNABII antibodies and HMGB1 each collapse biofilms, enabling the host’s immune response to naturally and rapidly clear large percentages of the NRel bacteria.
“When you mix them together, the biofilm infection is gone — there are no detectible bacteria at all,” says Dr. Goodman. The function of HMGB1 and its combined effects with DNABII antibodies have been tested and confirmed in multiple animal models.
Dr. Goodman believes that understanding how and why the immune system largely avoids fighting biofilms could hold valuable insights both for improving biofilm-based therapeutics and for managing life-threatening infections. Controlling how many bacteria are released and when, as well as how much inflammation arises in response — enough to recruit help from the immune system but not enough to trigger sepsis — could be a game-changer in clinical care.
“When you mix [DNABII antibodies and HMGB1] together, the biofilm infection is gone — there are no detectible bacteria at all.”
— Steven Goodman, PhD

The Future of Biofilm-Centered Therapies
Biofilms have the potential to recalibrate the global struggle against antimicrobial resistance, provide new tools in regulating the gut’s microbiome and even transform the efficacy of over-the-counter probiotic products for general wellness.
Positioned at the cusp of this medical revolution, the research teams at Nationwide Children’s continue to pour their time, effort and intellect into biofilm management, with ever-advancing goals in mind — new clinical trials, grants to study new diseases, new patents, and new discoveries in biofilm biology.
“It’s really fun to see how you can move so well and so quickly when you have people who challenge each other and aren’t just here to say ‘good idea,’” says Dr. Bakaletz of the team’s progress in the field over the past few decades. “We still have bad days when things just didn’t work out. But we don’t quit, we keep our eyes on the prize.”
This article appeared in the Fall/Winter 2024 issue. Download the full issue.
References:
- Al-Hadidi A, Navarro J, Goodman SD, Bailey MT, Besner GE. Lactobacillus reuteri in its biofilm state improves protection from experimental necrotizing enterocolitis. Nutrients. 2021 Mar 12;13(3):918.
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022 Feb 12;399(10325):629-655.
- Buzzo JR, Devaraj A, Gloag ES, Jurcisek JA, Robledo-Avila F, Kesler T, Wilbanks K, Mashburn-Warren L, Balu S, Wickham J, Novotny LA, Stoodley P, Bakaletz LO, Goodman SD. Z-form extracellular DNA is a structural component of the bacterial biofilm matrix. Cell. 2021 Nov 11;184(23):5740-5758.e17.
- Devaraj A, Novotny LA, Robledo-Avila FH, Buzzo JR, Mashburn-Warren L, Jurcisek JA, Tjokro NO, Partida-Sanchez S, Bakaletz LO, Goodman SD. The extracellular innate-immune effector HMGB1 limits pathogenic bacterial biofilm proliferation. Journal of Clinical Investigation. 2021 Aug 16;131(16):e140527.
- Duff AF, Jurcisek JA, Kurbatfinski N, Chiang T, Goodman SD, Bakaletz LO, Bailey MT. Oral and middle ear delivery of otitis media standard of care antibiotics, but not biofilm-targeted antibodies, alter chinchilla nasopharyngeal and fecal microbiomes. NPJ Biofilms and Microbiomes. 2024 Feb 3;10(1):10.
- Goodman SD, Bakaletz LO. Bacterial biofilms utilize an underlying extracellular DNA matrix structure that can be targeted for biofilm resolution. Microorganisms. 2022 Feb 18;10(2):466.
- Kurbatfinski N, Hill PJ, Tobin N, Kramer CN, Wickham J, Goodman SD, Hall-Stoodley L, Bakaletz LO. Disruption of nontuberculous mycobacteria biofilms induces a highly vulnerable to antibiotic killing phenotype. Biofilm. 2023 Nov 25;6:100166.
- Kurbatfinski N, Kramer CN, Goodman SD, Bakaletz LO. ESKAPEE pathogens newly released from biofilm residence by a targeted monoclonal are sensitized to killing by traditional antibiotics. Frontiers in Microbiology. 2023 Jul 26;14:1202215.
- Madison AA, Bailey MT. Stressed to the core: Inflammation and intestinal permeability link stress-related gut microbiota shifts to mental health outcomes. Biological Psychiatry. 2024 Feb 15;95(4):339-347.
- Novotny LA, Clements JD, Goodman SD, Bakaletz LO. Transcutaneous immunization with a Band-Aid prevents experimental otitis media in a polymicrobial model. Clinical and Vaccine Immunology. 2017;24:e00563-16.
- Olson JK, Rager TM, Navarro JB, Mashburn-Warren L, Goodman SD, Besner GE. Harvesting the benefits of biofilms: A novel probiotic delivery system for the prevention of necrotizing enterocolitis. Journal of Pediatric Surgery. 2016 Jun;51(6):936-41.
- Schmitt LM, Smith EG, Pedapati EV, Horn PS, Will M, Lamy M, Barber L, Trebley J, Meyer K, Heiman M, West KHJ, Hughes P, Ahuja S, Erickson CA. Results of a phase Ib study of SB-121, an investigational probiotic formulation, a randomized controlled trial in participants with autism spectrum disorder. Scientific Reports. 2023 Mar 30;13(1):5192.
- Wala SJ, Sajankila N, Ragan MV, Duff AF, Wickham J, Volpe SG, Wang Y, Conces M, Dumbauld Z, Purayil N, Narayanan S, Rajab A, Mihi B, Bailey MT, Goodman SD, Besner GE. Superior performance of biofilm versus planktonic Limosilactobacillus reuteri in protection of the intestines and brain in a piglet model of necrotizing enterocolitis. Scientific Reports. 2023 Oct 23;13(1):17740.
- Wang Y, Jaggers R, Shaffer T, Mar P, Galley J, Deshponde S, Rajab A, Mashburn-Warren L, Buzzo J, Goodman S, Bailey M, Besner G. Lactobacillus reuteri in its biofilm state promotes neurodevelopment after experimental necrotizing enterocolitis. Brain, Behavior & Immunity Health. 2021;14:100256.
- Wilbanks KQ, Mokrzan EM, Kesler TM, Kurbatfinski N, Goodman SD, Bakaletz LO. Nontypeable Haemophilus influenzae released from biofilm residence by monoclonal antibody directed against a biofilm matrix component display a vulnerable phenotype. Scientific Reports. 2023 Aug 10;13(1):12959.
Image Credits:
- Header image first published in, Goodman S, Obergfell K, Jurcisek J, Novotny LA, Downey JS, Ayala EA, Tjokro N, Li B, Justice SS, Bakaletz LO. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunology. 2011;4:625-637.
- Nationwide Children’s (portraits, except Dr. Hamilton, and illustration)
- Dr. Hamilton’s photo was provided by Dr. Hamilton and used with permission.
- Microscopy images provided by Drs. Bakaletz and Goodman, Nationwide Children’s.
About the author
Katherine (Katie) Brind’Amour is a freelance medical and health science writer based in Pennsylvania. She has written about nearly every therapeutic area for patients, doctors and the general public. Dr. Brind’Amour specializes in health literacy and patient education. She completed her BS and MS degrees in Biology at Arizona State University and her PhD in Health Services Management and Policy at The Ohio State University. She is a Certified Health Education Specialist and is interested in health promotion via health programs and the communication of medical information.
- Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
- Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
- Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
- Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 28, 2014









