The Collapse of Biofilms?
The Collapse of Biofilms? https://pediatricsnationwide.org/wp-content/uploads/2020/04/biofilm-header-1024x575.jpg 1024 575 Jeb Phillips Jeb Phillips https://pediatricsnationwide.org/wp-content/uploads/2021/03/Jeb-Phillips.jpg- April 28, 2016
- Jeb Phillips
Scientists are working to eliminate the causes of countless chronic and recurrent human infections.
Before the discoveries that could lead to biofilm eradication, before the idea that he was even working on treatments for the bacterial communities that are crucial to most human infections, Steven Goodman, PhD, had a mystery on his hands.
For nearly 15 years. Dr. Goodman, then a biochemist at the University of Southern California’s Herman Ostrow School of Dentistry, found a certain group of proteins outside the cells of the Streptococcus mutans bacterium in 1994. He didn’t expect that.
S. mutans is one of the primary causative agents of tooth decay. He was studying the DNABII proteins inside the bacteria — the proteins help create the architecture of the cell’s DNA. But he had found the proteins outside, too. What could they be doing there?
“It was driving me nuts,” he says.
Over time, other researchers discovered that DNA was also outside of bacterial cells. So there was extracellular DNA (eDNA), and there were extracellular DNABII proteins that could help bend the eDNA. Still, no theory that Dr. Goodman had could tie them together.
Then came Dec. 4, 2008. Dr. Goodman attended a presentation by Lauren Bakaletz, PhD, director of the Center for Microbial Pathogenesis in The Research Institute at Nationwide Children’s Hospital. A vaccine she had developed targeted middle ear infections.
Organizers had asked Dr. Goodman to be a discussant — essentially, to pose questions about Dr. Bakaletz’s work after she spoke. But he didn’t ask a single question. He was stunned into silence. Because as part of her presentation, Dr. Bakaletz showed a slide to illustrate the focus of the vaccine.
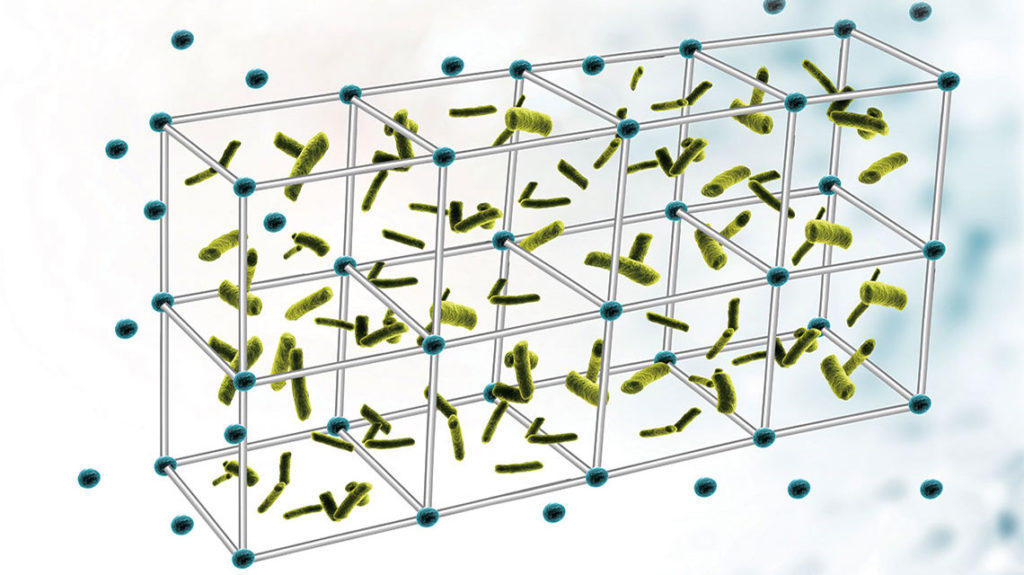
In the background, just for context, was a large eDNA structure stained blue. It looked like a lattice.
Dr. Bakaletz actually remembers saying as she showed the slide: “Ignore the blue, though I am interested in why the DNA is this perfect basketweave.”
And just like that, Dr. Goodman thought he had the solution to his mystery from 1994. That blue-stained structure was the extracellular matrix of a biofilm. He believed the slide was showing a huge mass of eDNA shaped into a basketweave by the same kind of extracellular proteins he had found so long before.
I almost fell off of my chair. If you are lucky, you get two or three ‘aha!’ moments in your career. This was one.
— Steven Goodman, PhD

Dr. Bakaletz, who is also a professor of Pediatrics and Otolaryngology at The Ohio State University College of Medicine, wanted to push the idea once he explained it to her. If they could prove how the extracellular matrix was put together, maybe they could collapse it. And if they could collapse a biofilm, a host of chronic microbial infections that are caused by bacterial biofilms — urinary tract infections, cystic fibrosis-associated infections, endocarditis, middle ear infections and many, many others — might be treatable in a way they aren’t now.
“If all of this worked out, I thought it was going to be the coolest thing I had ever seen,” Dr. Bakaletz says.
A Fortress for Bacteria
Biofilms are highly-organized communities of cells that are attached to a surface and encased in an extracellular polymeric substance or matrix. About 80 percent of bacteria live in biofilms instead of in a free-floating state, and the matrix protects the bacteria from harsh environmental elements such as the host’s immune system, antibiotics and shear force.
Biofilms form on human and animal tissue, but they can form on almost any other type of surface. They can be on catheters, implanted artificial joints, medical equipment and kitchen cutting boards, says Dr. Bakaletz. They foul water and oil pipes. They create drag on ship hulls and airplane wings.
Human and animal infections can be extraordinarily difficult to treat because of the protective matrix. In fact, the most effective treatment is mechanical removal of the biofilms. That’s possible with teeth and airplane wings. Not in a child’s middle ear or a cystic fibrosis patient’s lungs.
Beyond the protective matrix, there are other issues to consider, Dr. Bakaletz says. Bacteria in biofilms have slowed metabolisms and are no longer dividing, so antibiotics that work only on dividing cells are ineffective. And biofilms are rarely homogenous; a single biofilm is most often a consortium of different bacteria interacting with each other. The various etiologies make it difficult to knock out all of the bacteria with a single treatment.
What does appear to be consistent among many different biofilms, however, is the structure of the extracellular matrix and the way DNABII proteins work to hold the eDNA together for that matrix. The extracellular proteins that Dr. Goodman found in 1994 with S. mutans bacteria — the contributor to tooth decay — were the same kind of proteins that Drs. Bakaletz and Goodman found nearly 15 years later with nontypeable Haemophilus influenzae, a common cause of middle ear infection.
“If all of these bacteria are going to interact with one another under the same shield, then that shield needs to have the same structure,” Dr. Goodman says. “What is the biological material they all have in common? We argue it’s DNA and these proteins. It’s akin to steel girders in a building. The building can have rooms made of wood or of plaster or whatever else, but the steel girders are the same. The proteins are the Esperanto of structural material. It’s the language every bacterium speaks.”
The proteins stabilize the biofilm matrix. Take away the proteins, and the biofilm could collapse.

If all of this worked out, I thought it was going to be the coolest thing I had ever seen.
— Lauren Bakaletz, PhD
Collapsing the Matrix
Before that important day in 2008, Dr. Goodman had developed an antibody to the DNABII proteins from the bacteria E. coli. The antibody recognized the proteins; Dr. Bakaletz used it in 2009 to show the proteins were at the vertices of the H. influenzae biofilm matrix. The researchers hypothesized that if they introduced the antibody while a biofilm was forming, the antibody would soak up the proteins and prevent the formation.
Dr. Bakaletz tried an even more robust experiment – she added the antibodies to an established biofilm. It should not have worked, Dr. Goodman points out. Conventional wisdom is that antibodies cannot penetrate a biofilm. So they should have no effect on an established biofilm. But they did.
“It was amazing,” Dr. Bakaletz says. “Biofilms were collapsing left and right. They were collapsing in vitro and in vivo. When a biofilm collapses in a body site and the bacteria are cleared, it means the disease is cured. We said to ourselves, ‘we have to run with this.’”
Dr. Goodman eventually joined Dr. Bakaletz at Nationwide Children’s as a principal investigator in the Center for Microbial Pathogenesis. The pair has since shown that while the antibodies do not pull proteins out of biofilms, they do titrate off the proteins that occasionally detach. Those proteins cannot rejoin the extracellular matrix. When enough proteins have detached and are unable to reattach, the matrix crumbles.
That has proven to be true in every biofilm that the researchers have tested, but Drs. Bakaletz and Goodman are now working on a study about a kind of exception they have found. Proteins from the bacterium Porphyromonas gingivalis, which causes gum disease, are recognized by a different antibody. That antibody is selective for the P. gingivalis protein and can disperse P. gingivalis biofilms, but it does not affect other bacterial biofilms. This suggests that it is possible to target harmful biofilms while leaving helpful ones intact.
“There are good bacteria and biofilms in our body. The gut has biofilms that are healthy for most of us,” Dr. Bakaletz says. “You don’t want to disrupt those. You do want to eradicate the most pathogenic ones if you can.”
Other Directions
Scientists all over the world are working on the issue of biofilm infections. Preventing their formation with anti-adhesive surfaces and attacking young biofilms with antibiotic combinations are some of the strategies seen as having the greatest potential. Established biofilms, like the ones Drs. Bakaletz and Goodman work on, can be more resistant to treatment.
Some of the most promising recent research on eradicating established biofilms has come from the lab of Robert W. Huigens III, PhD, an assistant professor in the College of Pharmacy at the University of Florida. Dr. Huigens’ research team designs, chemically synthesizes and evaluates novel small molecules against biofilms, with the goal of bringing a biofilmeradicating agent to the clinic for therapeutic use. Dr. Huigens has found that a group of compounds called halogenated phenazines (HP), which are inspired by a marine antibiotic, can eradicate methicillin-resistant Staphylococcus aureus (MRSA) and other biofilms.
“The mechanism by which these compounds kill biofilm cells is not entirely clear,” Dr. Huigens says. “But as an alternative to antibodies and proteins, these HP small molecules infiltrate biofilms and kill the persistent bacteria inside.”
Unlike some other biofilm-eradicating agents, HPs do not “punch holes” in cellular membranes. HP small molecules show selectivity toward bacterial cells over healthy human cell types, such as red blood cells.
Dr. Huigens, like Dr. Goodman, says that one of the most vexing issues with biofilm eradication is killing the bad while leaving the good. Previously discovered compounds have not made the distinction, but Dr. Huigens says that the HP compounds do.
“We have made an important first step in discovering and developing potent biofilm-killing agents,” Dr. Huigens says. “From here, it’s important to figure out exactly what is happening with these HP compounds. This will allow many different scientists with different expertise to work on this major problem too. This discovery has the potential to change people’s lives, as it is estimated that 17 million Americans are affected by biofilm infections each year.”
Long-Term Impact
That is ultimately what excites Drs. Bakaletz and Goodman at Nationwide Children’s as well: the huge impact this biofilm research could have on so many aspects of human life.
“We hope to eradicate biofilm infections, but there are other applications,” Dr. Goodman says. “It can be hard to find a biofilm infection in a person. Well, the antibodies can recognize biofilms, so they can be used as a diagnostic. They could recognize biofilms on food preparation surfaces and medical equipment and determine if they have been properly cleaned.”
Many questions remain, Dr. Bakaletz says. Do these antibodies work on every organism? What are the restrictions and parameters? How do we best deliver treatment? How often? Every day or just once?
All of those questions await more research.
When Drs. Bakaletz and Dr. Goodman start to talk about it, though, they lean forward and their words come fast. In some ways, they seem as excited as they were on that day in 2008, when Dr. Goodman saw a blue-stained matrix and almost fell off his chair.
We’re trained to be negative and skeptical. We’re trained to ask question after question. But after a while, we just had to look at each other and say, ‘this keeps on working.’
— Dr. Lauren Bakaletz
ProclarRx
The biofilm discoveries from Drs. Bakaletz and Goodman have spawned a biotechnology company, ProclaRx. Initially called Lattice Biotech when it was formed in 2014, ProclaRx wants to bring the biofilm matrix collapse work done at Nationwide Children’s to the marketplace.
Drs. Bakaletz and Goodman are co-founders, co-chairs and scientific advisors to ProclaRx. The executive leadership is comprised of other scientists with long histories in the biotech field, and Nationwide Children’s Office of Technology Commercialization has facilitated the company’s creation.
“We are very excited about the impact that this technology could have in the treatment of chronic infections and ProclaRx is an ideal partner driving the technology forward on this front,” says Matt McFarland, RPh, PhD, director of the Office of Technology Commercialization. “We also recognize that this is a platform technology and may have significant implications in industries far beyond health care.”
By early 2016, ProclaRx had secured $5.5 million in investments. The company would like to begin initial clinical trials by the end of this year, according to Dr. Bakaletz.
This article appeared in the Spring/Summer 2016 print issue. Download a PDF copy.
References:
- Goodman SD, Obergfell KP, Jurcisek JA, Novotny LA, Downey JS, Ayala EA, Tjokro N, Li B, Justice SS, Bakaletz LO. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunology. 2011 Nov;4(6):625-637.
- Brockson ME, Novotny LA, Mokrzan EM, Malhotra S, Jurcisek JA, Akbar R, Devaraj A, Goodman SD, Bakaletz LO. Evaluation of the kinetics and mechanism of action of anti-integration host factor-mediated disruption of bacterial biofilms. Molecular Microbiology. 2014 Sep;93(6):1246-1258.
- Garrison AT, Abouelhassan Y, Kallifidas D, Bai F, Ukhanova M, Mai V, Jin S, Luesch H, Huigens RW 3rd. Halogenated phenazines that potently eradicate biofilms, MRSA persister cells in non-biofilm cultures, and Mycobacterium tuberculosis. Angewandte Chemie International Edition. 2015 Dec 1;54(49):14819-14823.
Image credits: Nationwide Children’s
About the author
Jeb is the Managing Editor, Executive Communications, in the Department of Marketing and Public Relations at Nationwide Children's Hospital. He contributes feature stories and research news to PediatricsOnline, the hospital’s electronic newsletter for physicians and other health care providers, and to Pediatrics Nationwide. He has served as a communications specialist at the Center for Injury Research and Policy at The Research Institute and came to Nationwide Children’s after 14-year career as daily newspaper reporter, most recently at The Columbus Dispatch.
-
Jeb Phillipshttps://pediatricsnationwide.org/author/jeb-phillips/October 13, 2015
-
Jeb Phillipshttps://pediatricsnationwide.org/author/jeb-phillips/
-
Jeb Phillipshttps://pediatricsnationwide.org/author/jeb-phillips/November 24, 2015
-
Jeb Phillipshttps://pediatricsnationwide.org/author/jeb-phillips/January 19, 2016
- Posted In:
- Features