Profiling the Tumor Microenvironment Offers New Insight for Hard-to-Treat Tumors
Profiling the Tumor Microenvironment Offers New Insight for Hard-to-Treat Tumors https://pediatricsnationwide.org/wp-content/uploads/2022/07/AdobeStock_94289759-for-web-1024x269.jpg 1024 269 Katie Brind'Amour, PhD, MS, CHES Katie Brind'Amour, PhD, MS, CHES https://pediatricsnationwide.org/wp-content/uploads/2021/03/Katie-B-portrait.gif- August 18, 2022
- Katie Brind'Amour, PhD, MS, CHES
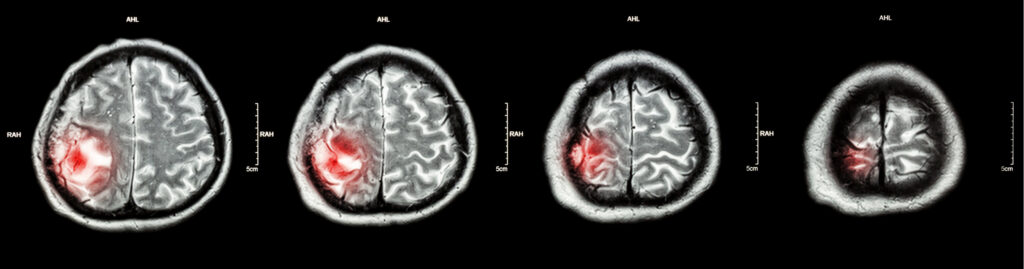
A multi-faceted approach to precision medicine may allow clinicians to gain a better understanding of cancer and the complex ecosystem in which it resides.
The field of precision medicine has evolved in leaps and bounds in the past two decades. Genomic profiling tools that were once cost-prohibitive and slow are now reasonably affordable and fast enough to incorporate into standard clinical care for oncology patients. But according to one principal investigator in the Steve and Cindy Rasmussen Institute for Genomic Medicine (IGM) at Nationwide Children’s Hospital, it seems that even those advancements are falling short for some of the most challenging malignancies.
Prajwal Rajappa, MD, MS, director of the Translational Neuro-Oncology Laboratory at IGM, actively advocates for precision medicine to expand profiling efforts to include factors outside of the tumor — such as immune cells both resident to the tumor and recruited and reprogrammed nearby, or immune cells circulating in the blood — which he believes could offer the additional insights clinician researchers need to make significant headway against multiply recurrent, inoperable and metastatic cancers.
“We know now that the tumor lives in a complex microenvironment,” says Dr. Rajappa, who recently was invited to present on the topic at Duke University, MD Anderson Cancer Center and Cleveland Clinic, among other institutions. “This ecosystem is dynamically evolving over the course of disease progression, as well as in response to the treatment paradigm. Precision medicine must also evolve to reflect our improved understanding of that ecosystem.
His research has already demonstrated the advantage of a robust profiling strategy for pediatric patients with central nervous system (CNS) tumors, with direct implications for clinical management, in Neuro-Oncology Advances.
To add to the argument for expanding precision medicine to include extrinsic factors within the microenvironment, some of Dr. Rajappa’s findings from tumor-extrinsic single-cell RNA sequencing, transcriptomic profiling and immune cell cluster analyses suggest a potential limitation in our current standard of care for patients with high-grade gliomas (HGG).
“First line immunotherapies we currently use in standard HGG therapy are largely focused on improving T-cell function by targeting checkpoint molecules,” says Dr. Rajappa. “Unfortunately, in the tumor-extrinsic compartment in HGG, there are a robust population of bone marrow-derived myeloid cells that infiltrate the local tumor microenvironment and potentiate immunosuppression, limiting the trafficking and activation of cytotoxic T-cells and natural killer [NK] cells that can get to and destroy tumor cells.”
Thus, the potential of these immunotherapies can’t be realized; there are very few immune cells in the tumor microenvironment for them to significantly impair tumor growth. However, it is possible that improved understanding of nuances like this one could offer opportunities to bolster and improve first-line treatment immunotherapy and standard of care treatment approaches.
For example, Dr. Rajappa’s research has revealed that genes that suppress and inactivate T-cells are upregulated in infiltrating myeloid cells in HGG. Modulating those genes to impair immunosuppression could potentially help address the local tumor microenvironment immune cell shortage and increase anti-tumor activity in response to checkpoint inhibitors.
“It’s already clear that tumor-intrinsic approaches can only take us so far in terms of treatment efficacy for malignant, multiply recurrent and inoperable CNS tumors,” says Dr. Rajappa. “We need to leverage advanced next-generation sequencing approaches for those different compartments within the microenvironment and define the clinical utility of longitudinal liquid biopsy to evaluate disease progression and treatment response. A multimodal approach has the added benefit of providing a temporal data set, which will be critical to our understanding of what therapies may be most effective prior to or during the evolution of tumor progression.”
His lab has research underway investigating whether liquid biopsies can identify biomarkers such as cytokines, chemokines and circulating tumor cells that may offer information on tumor prognosis and response to treatment.
References:
- Rajappa P. Single-cell RNA Sequencing Reveals Immunosuppressive Myeloid Cell Diversity During Malignant Progression in Glioma. Grand Rounds Presentation at Duke University, 20 April 2022.
- Rajappa P, Eng KW, Bareja R, Bander ED, Yuan M, Dua A, Bhanu Maachani U, Snuderl M, Pan H, Zhang T, Tosi U, Ivasyk I, Souweidane MM, Elemento O, Sboner A, Greenfield JP, Pisapia DJ. Utility of multimodality molecular profiling for pediatric patients with central nervous system tumors. Neuro-Oncology Advances. 2022 Mar 10;4(1):vdac031.
About the author
Katherine (Katie) Brind’Amour is a freelance medical and health science writer based in Pennsylvania. She has written about nearly every therapeutic area for patients, doctors and the general public. Dr. Brind’Amour specializes in health literacy and patient education. She completed her BS and MS degrees in Biology at Arizona State University and her PhD in Health Services Management and Policy at The Ohio State University. She is a Certified Health Education Specialist and is interested in health promotion via health programs and the communication of medical information.
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 28, 2014