Encouraging First Report of Systemic Delivery of Micro-dystrophin Gene Therapy in Children With Duchenne Muscular Dystrophy
Encouraging First Report of Systemic Delivery of Micro-dystrophin Gene Therapy in Children With Duchenne Muscular Dystrophy https://pediatricsnationwide.org/wp-content/themes/corpus/images/empty/thumbnail.jpg 150 150 Abbie Miller Abbie Miller https://pediatricsnationwide.org/wp-content/uploads/2023/05/051023BT016-Abbie-Crop.jpgOne-year data from the first four patients to receive a single dose of the rAAVrh74.MHCK7.micro-dystrophin gene therapy is published in JAMA Neurology.
Researchers from Nationwide Children’s Hospital have published in JAMA Neurology results from the first four patients treated in the first clinical trial of systemic delivery of micro-dystrophin gene therapy in children with Duchenne muscular dystrophy (DMD) – and initial findings suggest that the therapy can provide functional improvement that is greater than that observed under the standard of care.
“We are very pleased to report successful delivery of the micro-dystrophin transgene to the nuclei – corresponding to robust gene expression and proper localization of micro-dystrophin. This coincides with improvements in functional measurements in study participants who received SRP-9001,” says Jerry Mendell, MD, the study’s co-author and principal investigator with the Center for Gene Therapy in the Abigail Wexner Research Institute at Nationwide Children’s Hospital.
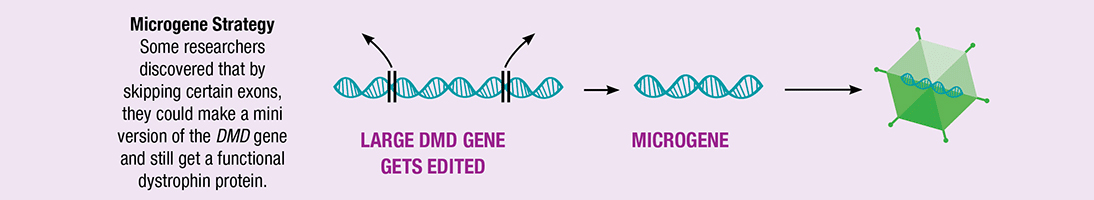
DMD is a fatal neuromuscular disease that occurs in approximately one in every 5,000 males worldwide. It is caused by a mutation in the gene that encodes for dystrophin, and symptoms usually appear in infants and toddlers. The dystrophin gene itself is too large to fit in to the adeno-associated viral vector used in the gene therapy technology utilized by the study. Researchers have developed micro-dystrophin as a microgene that provides function while still fitting in the vector.
Four ambulatory participants, aged 4 to 7 years at time of infusion were treated with a single dose of 2.0 x 1014 vg/kg rAAVrh74.MHCK7.micro-dystrophin (SRP-9001 micro-dystrophin, Sarepta Therapeutics), which was infused through a peripheral limb vein. All treatment-related events were mild to moderate and there were no serious adverse events. The most common treatment-related adverse event was vomiting (9 of 18 events). Three patients had temporarily elevated levels of gamma-glutamyltransferase, which was resolved with corticosteroids.
At 12 weeks post-infusion, gastrocnemius muscle biopsy specimens showed a mean of 81.2% of muscle fibers expressing micro-dystrophin, with a mean intensity of 96% at the sarcolemma. Western blot showed a mean expression of 74.3% without fat or fibrosis adjustment and 95.8% with adjustment.
All of the participants had confirmed vector transduction and showed functional improvement of North Star Ambulatory Assessment (NSAA) scores. The NSAA is a 17-item measure of ambulatory functions with a score range from 0 to 34.
Change in NSAA Scores 1 Year Post-infusion
| Participant | Age (years) | NSAA (baseline) | NSAA (1 y post-infusion) | Change in NSAA |
| 1 | 5 | 18 | 25 | 7 |
| 2 | 4 | 19 | 27 | 8 |
| 3 | 6 | 26 | 28 | 2* |
| 4 | 4 | 19 | 24 | 5 |
* At age 6, scores would be expected to decrease, so a gain of 2 points might be under-representing improvement, according to the authors.
All participants also showed reductions in creatine kinase (CK) levels that were maintained for one year.
Other functional outcomes measured included time to rise, four-stair climb, 100-m timed test and handheld dynamometry for knee extensors and flexors and elbow extensors and flexors, but their results were more varied.
“NSAA score improvement and CK score reduction appear to be the most sensitive measures,” says Dr. Mendell. “Different magnitudes of improvement were observed in the other functional measurements, suggesting that a larger sample size and clinical trial are needed to validate improved motor function. Variation in clinical outcomes were associated with many factors, including age and disease severity.”
The study is intended to continue until March 2021.
“Duchenne muscular dystrophy is difficult to treat, and gene therapy offers a needed option having the potential to alter the course of the disease,” says Dr. Mendell. “Using currently available treatments, the condition is universally fatal, and patients usually succumb to the disease in their twenties.”
The safety results, together with biological and clinical markers of efficacy, provide proof-of-concept support for continuation of clinical trials for assessment of SRP-9001 using single-dose gene therapy in participants with Duchenne, he adds.
“Following the 9-month update we shared last year, the publication of these results further supports the potential for SRP-9001 to provide clinically meaningful functional improvements in terms of speed and magnitude for DMD patients,” says Louise Rodino-Klapac, PhD, senior vice president of gene therapy at Sarepta Therapeutics. “We look forward to advancing our ultimate goal of profoundly improving the lives of as many patients living with DMD as possible.”
Sarepta has exclusive rights to the gene therapy program initially developed at the Abigail Wexner Research Institute at Nationwide Children’s Hospital.
About the author
Abbie (Roth) Miller, MWC, is a passionate communicator of science. As the manager, medical and science content, at Nationwide Children’s Hospital, she shares stories about innovative research and discovery with audiences ranging from parents to preeminent researchers and leaders. Before coming to Nationwide Children’s, Abbie used her communication skills to engage audiences with a wide variety of science topics. She is a Medical Writer Certified®, credentialed by the American Medical Writers Association.
- Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
- Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
- Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
- Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/