Fighting Fibrosis
Fighting Fibrosis https://pediatricsnationwide.org/wp-content/uploads/2015/04/FightingFibrosis.jpg 1024 575 Katie Brind'Amour, PhD, MS, CHES Katie Brind'Amour, PhD, MS, CHES https://pediatricsnationwide.org/wp-content/uploads/2021/03/Katie-B-portrait.gif- April 25, 2015
- Katie Brind'Amour, PhD, MS, CHES
Fibrosis is an unmet medical challenge with no satisfactory test and insufficient therapy. Now, one naturally occurring cellular component could simultaneously diagnose and heal patients.
It’s much more than a million-dollar idea.
The person who invents a simple blood or urine test that can accurately measure the severity of scarring in internal organs will have developed a diagnostic and clinical monitoring tool to help hundreds of thousands of people — adults and children alike — avoid risky biopsies and expensive imaging tests each year.
Even more dramatic will be the discovery of a therapy that cures fibrosis, the formation of scar tissue that, in excess, can interfere with organ function. Although this scarring may subside in some individuals after the underlying disease or problem is addressed, there are many others whose bodies cannot dissolve the fibrous tissue and replace it with healthy cells.
Bringing a cure to people who otherwise may be destined for organ transplant would make a lot of happy patients. And a much shorter organ donation wait list.
After all, scarring is a very common problem.
From the fine line that remains after a small cut to the expansive, collagen-rich matrix that is a feature of organ damage caused by long-term disease, there are few individuals who have not experienced the biological phenomenon to some degree. And in certain organ systems, the problem is a growing one.
More than 100 liver diseases and a wide variety of other medical complications can cause liver fibrosis in children. Some of these are anatomical defects, such as biliary atresia or missing bile ducts, and some are the result of chronic illness, drugs or even poor diet.
Most pediatric health professionals are no strangers to the quickly increasing rates of obesity among U.S. children. What fewer people may know is that rates of liver complications, such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis, are rising with the excess weight. These conditions result from excessive fatty deposits accumulating in the liver, which lead to inflammation and liver damage and, in some patients, fibrosis and cirrhosis.
Estimates suggest that as many as one in every 10 U.S. children have fatty livers, a major risk factor for developing future liver fibrosis.
An accurate liver fibrosis diagnostic test — and a cure — cannot come soon enough.
Now, the research of David R. Brigstock, PhD, principal investigator in the Center for Clinical and Translational Research in The Research Institute at Nationwide Children’s Hospital, may have identified a striking way to resolve both diagnostic and therapeutic challenges with one molecular answer: exosomes.
BUILDING A BETTER BLOOD TEST
Although dangerous internal scarring affects millions of U.S. children and adults, there is currently no accurate, noninvasive way to assess this pathology. Biopsies are the gold standard for measuring liver fibrosis, but this approach is subject to errors in sampling and is highly invasive, carrying rare but significant risks of complications and presenting obstacles for patients who need to be tested repeatedly.
Imaging technologies, including ultrasound, elastography and MRI, are evolving rapidly and have the advantage of being noninvasive. But readings may not provide the desired discrimination and can be skewed by the presence of other liver pathologies. The expense and time-consuming nature of such procedures accentuate the need for a simple biomarker-based blood or urine test.
But it wasn’t this clinical objective that Dr. Brigstock and his three-person team initially intended to achieve.
Their past research focused on the body’s mechanisms for regulating the production of connective tissue growth factor, which is instrumental in the production of scar-forming collagen. They changed course upon their discovery that exosomes are produced by the key fibrosis-inducing liver cells, called hepatic stellate cells, and have since devoted their work to understanding how exosomes package and deliver molecular information about cell activity to other liver cells.
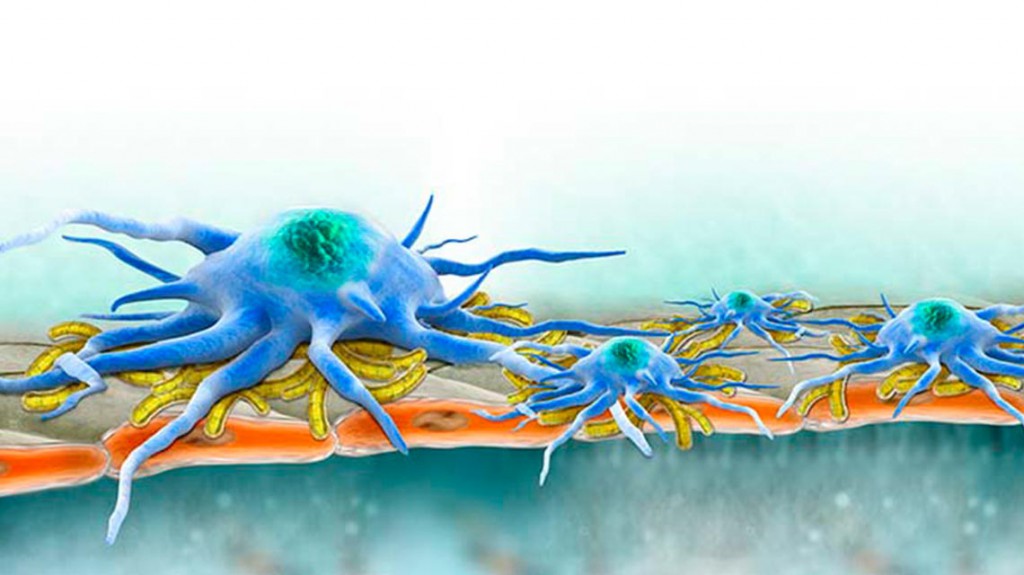
Exosomes are tiny vesicles that carry molecular information from cell to cell, serving as communicators and, perhaps, instigators. Many human cell types pack exosomes with a complex molecular payload that is a snapshot of their own activity. Exosomes carrying micro-RNA (miRNA), messenger RNA (mRNA) and proteins are ejected from the cell and then taken up by other cells whose behavior is modified according to the molecular messages received.
Exosomes secreted by liver cells don’t just take up residence in the liver. They travel, and some make their way into the bloodstream — hence Dr. Brigstock’s idea for a novel blood test to indicate the presence and severity of liver fibrosis.
“If we can ‘read’ the molecular information being conveyed in exosomes, we may be able to filter out the molecular messages that relate to liver fibrosis and use them as biomarkers for assessing different levels of scarring,” says Dr. Brigstock, who also is a member of the Department of Pediatric Surgery at Nationwide Children’s and a professor of surgery at The Ohio State University College of Medicine. “Theoretically, the concept could be applied to any organ, since the process of fibrosis and collagen production is very similar throughout the body.”
Dr. Brigstock believes that the molecular information carried by exosomes may include signals that can be used to track them to a specific injury site in the body. Further changes in that information could theoretically offer indicators of increasing or decreasing injury. The key is learning to read the signals.
“We are desperate to know what’s going on in the liver,” says Scott L. Friedman, MD, chief of the Division of Liver Diseases and dean for Therapeutic Discovery at the Icahn School of Medicine at Mount Sinai. Dr. Friedman discovered the hepatic stellate cells that release the exosomes identified in Dr. Brigstock’s work. “David’s research is among the most exciting of its type anywhere.”
Developing accurate blood tests for fibrosis is not without its challenges, however.
Pediatric liver fibrosis expert Ronald J. Sokol, MD, section head of Gastroenterology, Hepatology and Nutrition at the University of Colorado (Denver) School of Medicine, is quick to point out that many attempts at blood tests to detect specific degrees of liver fibrosis have also looked promising — and ultimately fallen short of clinical needs.
“The study of exosomes and microparticles is a very hot area in science now,” says Dr. Sokol, who also serves as the associate medical director of UC Denver’s Pediatric Liver Center and Liver Transplantation Program. “Lots of researchers are examining it in cancer and other biologies, and it’s something that you can measure very nicely in the lab. To extrapolate that finding into a blood test for fibrosis is challenging, particularly when so many other biomarker-based blood tests have failed to pan out.”
Even so, he cannot deny the appeal of such a test and genuinely hopes Dr. Brigstock succeeds.
“It’s every doctor’s dream to have a simple blood test that tells us how much scarring is in the liver, and nothing currently on the market is completely accurate,” Dr. Sokol explains. “Many approaches in pharmaceutical development are enormously promising in mice and then don’t pan out in humans. That doesn’t mean that this technology should not be explored — it should be explored. There are a lot of good ideas that turn out to be true as well.”
But Dr. Brigstock’s approach has a special appeal, according to Laura E. Nagy, PhD, a professor of molecular medicine at Cleveland Clinic Lerner College of Medicine and director of the Cleveland Alcohol Center. Dr. Nagy reached out to Dr. Brigstock to collaborate after hearing his invited lecture at her institution.
“Many other biomarker approaches focus on one or two indicators,” Dr. Nagy says. “But exosomes are packed with information, and I believe they could offer a much broader perspective on what’s going on in the body. The more information, the better.”
Later this year, Dr. Nagy’s pending project center grant from the National Institutes of Health and the National Institute on Alcohol Abuse and Alcoholism will facilitate Dr. Brigstock’s clinical research on human blood samples.
“There’s lots of work to be done,” Dr. Friedman says of Dr. Brigstock’s work. “And while it’s not ready for prime time yet, the ability to capture information circulating in the blood about the state of the liver is exactly the type of new approach we need.”
And remarkably, this new angle on fibrosis research is not limited to a potential noninvasive blood profile.
SCRATCHING THE SURFACE
It’s rare for biology to hand medical science a veritable Holy Grail with sufficient power to both diagnose and cure disease. But judging by early work in mice, Dr. Brigstock believes that exosomes may just have that sort of potential.
“Exosomes appear to be critical in telling other cells whether to increase or decrease their production of fibrosis-inducing molecules such as connective tissue growth factor,” says Dr. Brigstock. His team made this discovery, which was featured as the cover article in a 2014 issue of the journal Hepatology. “The molecular information packaged into exosomes changes dynamically according to the status of the cell that loaded it up and sent it off into the world. In turn, this information is conveyed to neighboring cells that respond according to the exosomal messages received.”
If a hepatic stellate cell is over-producing collagen, Dr. Brigstock theorizes, it’s likely packaging molecular information into its exosomes, which communicate this message to neighboring cells that then follow suit.
The same goes for liver cells that have suppressed their fibrotic activity. Presumably, cargo from their exosomes could potentially reprogram other cells to stop their collagen production.
“It’s a newly discovered mechanism by which fibrosis is being regulated,” Dr. Brigstock says. “And whenever you’re talking about molecular mechanisms, many components in those processes are theoretical points of therapeutic intervention.”
Unlike the diagnostic profile test for which Dr. Brigstock has already begun pursuing human blood samples, exosome therapies for liver fibrosis cannot readily be moved out of the realm of animal studies.
But what he’s seen in animal models has not disappointed. When Dr. Brigstock’s team recognized that mice with fibrosis produced exosomes containing different information than nonfibrotic, healthy mice, they put two and two together.
“The question was whether, if we administered exosomes from healthy mice into the circulation of fibrotic mice, the liver would receive some of this information and respond by becoming less fibrotic,” Dr. Brigstock says.It seemed like a long shot.
They injected exosomes from healthy mice into mice with induced liver fibrosis. Within a week, the animals’ fibrosis had disappeared.
“It was stunning,” Dr. Brigstock says.
While his team gathers additional data to move their exosome projects along, with the hope of securing additional NIH funding, the possibilities for exosome therapy seem wide open.
The question was whether, if we administered exosomes from healthy mice into the circulation of fibrotic mice, the liver would receive some of this information and respond by becoming less fibrotic.
— David R. Brigstock, PhD, Nationwide Children’s Hospital
A WINNING STRATEGY
Dr. Brigstock’s mouse models benefited from exosome therapy when it was delivered via a simple injection. Although he has no particular reason to assume one method will win out over any other, Dr. Brigstock does admire the simple elegance of exosomes as a directly donatable or culturable product.
He is not alone. The method is already being tested in a field quite different from liver fibrosis: oncology.
For years, the field of cancer research has been buzzing about exosomes and their role in intercellular communication — signaling other cells to produce more active immune cells, for instance.
As many as half of all cancer patients receiving stem cell transplants develop severe graft-versus-host disease, a deadly condition in which the recipient’s body is at war with the donor cells. And half of these patients do not respond to the standard corticosteroid treatment.
Convinced that some of the curative capacity of the stem cells used in these transplants was derived from healthy exosomes, clinician-scientists in Germany increased the expression of exosomes in these stem cells and repeatedly injected them into a patient with severe, intractable graft-versus-host disease.
The patient’s symptoms significantly improved.
Although this patient later succumbed to pneumonia, the case report demonstrated that cultured exosomes could be administered as a therapeutic. Dozens of similar therapeutic applications have since begun at hospitals across the globe, with several therapies now in Phase II clinical trials.
Beyond direct culture and injection, alternative methods exist that offer researchers multiple avenues for exosome therapies.
According to Dr. Brigstock, techniques could include packaging a drug or replicated material from healthy donors into exosomes for targeted delivery to fibrotic liver cells. Theoretically, the same concept could work for other organ systems in the same way. And a similar “packaging and delivery” process is already therapeutically used with liposomes.
“I like the concept that you might bioengineer exosomes and package them with a molecule you would design that would turn off the fibrosis-related genes,” Dr. Sokol says. He believes a targeting mechanism would improve the chance of success.
“Cells could recognize another engineered molecule on the exosomes’ surface and take them up to deliver the drug to a specific cell type.”
The approach has science on its side, Dr. Brigstock says.
“Exosomes are nature’s own delivery vehicles. They protect their contents from adverse extracellular environments and allow molecular information to be delivered to another cell,” he says. “That is what you want to do with drugs. The body wouldn’t even recognize them as foreign before the drug was delivered.”
Dr. Brigstock is understandably optimistic. It’s not just the promise of the exosomes that makes him so, but the prospect for significant clinical improvements for individuals with liver fibrosis and other scarring problems.
While curing the underlying chronic disease leads to resolution of fibrosis in some patients, these responders cannot be predicted ahead of time. Those who are unlucky enough to remain heavily scarred need options.
A COMMON GOAL
Promising drugs developed by groups across the country are now in clinical trials. Some target the collagen matrix and attempt to break up the fibrous cell networks. Other approaches aim to block molecular pathways that perpetuate the production of fibrotic tissue. These and additional endeavors swell the range of biomarker-based blood tests and imaging research that could significantly improve fibrosis diagnosis and measurement in the future.
Dr. Brigstock knows his own ideas still face many obstacles before they can be clinically tested. But he remains driven by the overarching goal of targeting scar tissue to detect — and reverse — fibrosis.
“The truth of the matter is that when you test these approaches in animal models, you don’t really get a sense of whether they’ll work in people,” he says. “Ideally, I’d like to see exosomal therapy proven in other models of fibrosis and then investigated in clinical trials. It’s not a difficult path to anticipate.”
Not difficult to anticipate, perhaps, but a challenge to execute. Dr. Brigstock and his team are already collaborating with fibrosis experts from Ohio, California, New York, Canada and China to open the road to additional federal funding.
Whether or not an accurate reading of exosomal information in humans is possible, the group will continue animal studies to examine the tiny packages’ potential for fibrosis treatment.
“It may be a difficult path to tread,” Dr. Brigstock says, “But I have an innovative and resourceful team working on this project. I think it’s the most exciting work that we’ve participated in during the nearly 25 years I’ve been at Nationwide Children’s.”
This story also appeared in the Spring/Summer 2018 print issue. Download a PDF copy.
References:
- Charrier A, Chen R, Chen L, Kemper S, Hattori T, et al. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery. 2014 Sep, 156(3):548-55.
- Chen L, Charrier A, Zhou Y, Chen R, et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014 Mar, 59(3):1118-29.
- [Art reference.] Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nature Clinical Practice, Gastroenterology & Hepatology. 2004 Dec, 1(2):98-105.
- Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, et al. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease.Leukemia. 2014 Apr, 28(4):970-3.
Image credits: Nationwide Children’s
About the author
Katherine (Katie) Brind’Amour is a freelance medical and health science writer based in Pennsylvania. She has written about nearly every therapeutic area for patients, doctors and the general public. Dr. Brind’Amour specializes in health literacy and patient education. She completed her BS and MS degrees in Biology at Arizona State University and her PhD in Health Services Management and Policy at The Ohio State University. She is a Certified Health Education Specialist and is interested in health promotion via health programs and the communication of medical information.
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 28, 2014
- Posted In:
- Features