Researchers Characterize Growth and Remodeling of Tissue-Engineered Blood Vessels
Researchers Characterize Growth and Remodeling of Tissue-Engineered Blood Vessels https://pediatricsnationwide.org/wp-content/uploads/2020/10/TEVG-Stenosis-.jpg 600 400 Lauren Dembeck Lauren Dembeck https://pediatricsnationwide.org/wp-content/uploads/2021/03/Dembeck_headshot.gif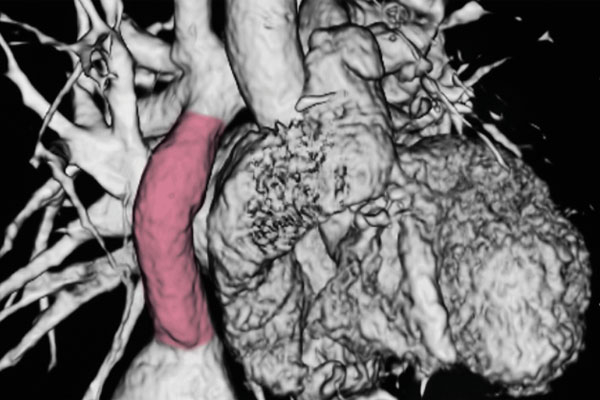
Tissue engineering may soon give babies with congenital heart disease new blood vessels capable of native function and growth.
Major cardiovascular reconstructive operations in babies with congenital heart defects require the use of artificial blood vessels called vascular grafts. Because these grafts are made of man-made materials, the recipients’ tissues eventually begin to outgrow them, contributing to significant morbidity and mortality in these children.
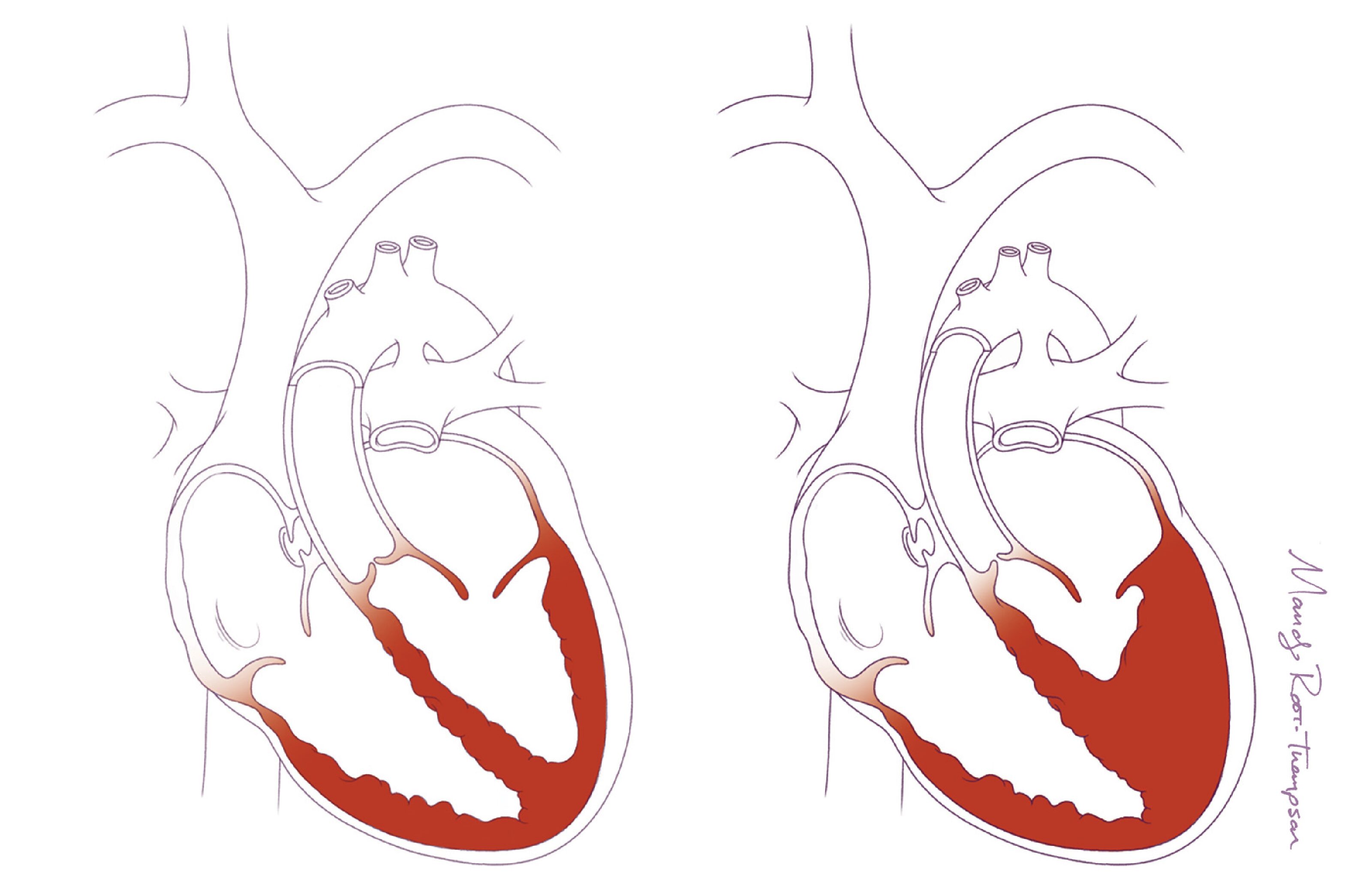
Tissue engineering represents a promising solution to this problem. The goal is to create biomaterials using a polymer scaffold and an individual’s own cells, referred to as tissue-engineered vascular grafts (TEVGs), that could potentially grow with the patient. As the child grows, the TEVG scaffold naturally degrades and transforms into a neovessel that mimics the function of a native blood vessel.
An international multidisciplinary collaboration led by physician-scientist Christopher Breuer, MD, of Nationwide Children’s Hospital has yielded new insights into in vivo TEVG remodeling with important implications for clinical use and future development of TEVGs for children with congenital heart disease. The findings were published in Communications Medicine.
“We have been able to take this technology from bench to bedside, and during one of our clinical trials, we observed a high incidence of graft narrowing early after implantation, which is something that we had not experienced in the preclinical and pilot studies. The patients were doing well, but their grafts were narrowed,” explains Dr. Breuer, who is director of the Center for Regenerative Medicine at Nationwide Children’s.
To understand the processes underlying this unexpected TEVG stenosis, Dr. Breuer and colleagues used an integrative computational-experimental approach to evaluate the natural history of neovessel formation in a preclinical model, combining in vitro accelerated degradation studies and mechanical testing, implantation studies, in vivo imaging and histology, and generating data-informed computational models of neovessel growth and remodeling.
“This iterative computational-experimental approach, where we collect experimental results, computationally model them, then predict what the next set of experiments should be, gives us the ability to rationally design experiments and narrow the search space for optimizing the TEVGs, instead of doing an infinite number of experiments,” added Dr. Breuer, who is also director of Tissue Engineering at The Ohio State University Center for Regenerative Medicine and Cell Based Therapies. “This is probably our best study to date describing the broad growth potential of TEVGs.”
The computational modeling can account for evolving changes in TEVG diameter and thickness, composition, and material properties due to immuno- and mechano-mediated stimuli, as well as complex hemodynamics and changing fractions of polymer and cells.
With modeling, the researchers predicted that the structural integrity of the polymeric scaffold would be lost over the first 26 weeks in vivo but that polymeric fragments would persist for up to 52 weeks. Their models indicated that the early neovessel tissue growth would be driven primarily by inflammatory processes in response to the grafts’ polymeric scaffold and that, as the scaffold degrades, neovessel remodeling would progressively become mediated by mechanobiological processes, such as blood flow-induced wall shear stress and pressure-induced intramural stress.
“In the preclinical model, we confirmed that early neovessel formation is driven primarily by the foreign body reaction to the scaffold, leading to an early period of remodeling characterized by transient TEVG stenosis,” said Kevin Blum, MD-PhD Candidate in the Breuer laboratory and first author of the study.
The team also observed that as the scaffold degrades, mechano-mediated neotissue remodeling became dominant, as predicted by the model, around 26 weeks. After the scaffold was completely degraded, the resulting neovessels continued to grow and remodel in a manner that mimics the behavior of native vessels, including biological growth capacity.
“We are constantly striving to bring our discoveries to the clinic to help our patients, and this experimental-computational approach has proved valuable and is accelerating that progress,” said Dr. Breuer.
Reference
Blum KM, Zbinden JC, Ramachandra AB, Lindsey SE, Szafron JM, Reinhardt JW, Heitkemper M, Best CA, Mirhaidari GJM, Chang YC, Ulziibayar A, Kelly J, Shah KV, Drews JD, Zakko J, Miyamoto S, Matsuzaki Y, Iwaki R, Ahmad H, Daulton R, Musgrave D, Wiet MG, Heuer E, Lawson E, Schwarz E, McDermott MR, Krishnamurthy R, Krishnamurthy R, Hor K, Armstrong AK, Boe BA, Berman DP, Trask AJ, Humphrey JD, Marsden AL, Shinoka T, Breuer CK. Tissue engineered vascular grafts transform into autologous neovessels capable of native function and growth. Commun Med (Lond). 2022 Jan 10;2:3. doi: 10.1038/s43856-021-00063-7. PMID: 35603301; PMCID: PMC9053249.
Image credit: Nationwide Children’s
About the author
Lauren Dembeck, PhD, is a freelance science and medical writer based in New York City. She completed her BS in biology and BA in foreign languages at West Virginia University. Dr. Dembeck studied the genetic basis of natural variation in complex traits for her doctorate in genetics at North Carolina State University. She then conducted postdoctoral research on the formation and regulation of neuronal circuits at the Okinawa Institute of Science and Technology in Japan.
- Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
- Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
- Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
- Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/January 29, 2019