Taking Innovation to Heart: Next Gen Interventions in Heart Valve Disease
Taking Innovation to Heart: Next Gen Interventions in Heart Valve Disease https://pediatricsnationwide.org/wp-content/uploads/2017/09/Cover-Final-Flat-CMYK-header-for-web-1024x575.gif 1024 575 Abbie Miller Abbie Miller https://pediatricsnationwide.org/wp-content/uploads/2023/05/051023BT016-Abbie-Crop.jpg- September 12, 2017
- Abbie Miller
From bioengineers to interventional cardiologists, molecular biologists to cardiothoracic surgeons, experts with diverse backgrounds are focusing on the problem of heart valve disease in children.
Heart valve disease affects more than 5 million Americans. And while acquired disease in the adult population certainly accounts for much of this, children with heart valve disease face multiple surgeries, procedures and morbidities over the course of their lives.
Pediatric heart valve disease can present at any age. Some infants have valves that need to be treated early on. Some children with bicuspid aortic valves may not even know they have a congenital heart defect until adolescence or adulthood. Each case of congenital heart valve disease is unique, making standardized treatments more difficult to come by.
Heart valve replacement is lifesaving but it is invasive. And the associated morbidity and mortalities of existing treatments for heart valve disease are trade-offs. Despite efforts to treat symptoms with medication or repair the valve through surgery, treatment for children often arrives at heart valve replacement.
Now, surgeons, tissue engineers and interventional cardiologists are tackling heart valve replacements with an aim to improve the outcomes for these children. And molecular biologists, geneticists and engineers are working to understand how these heart defects form in the first place. With rapidly advancing science and technology, and a spirit of collaboration and innovation among scientists, big changes are on the horizon for the millions of patients with heart valve disease.
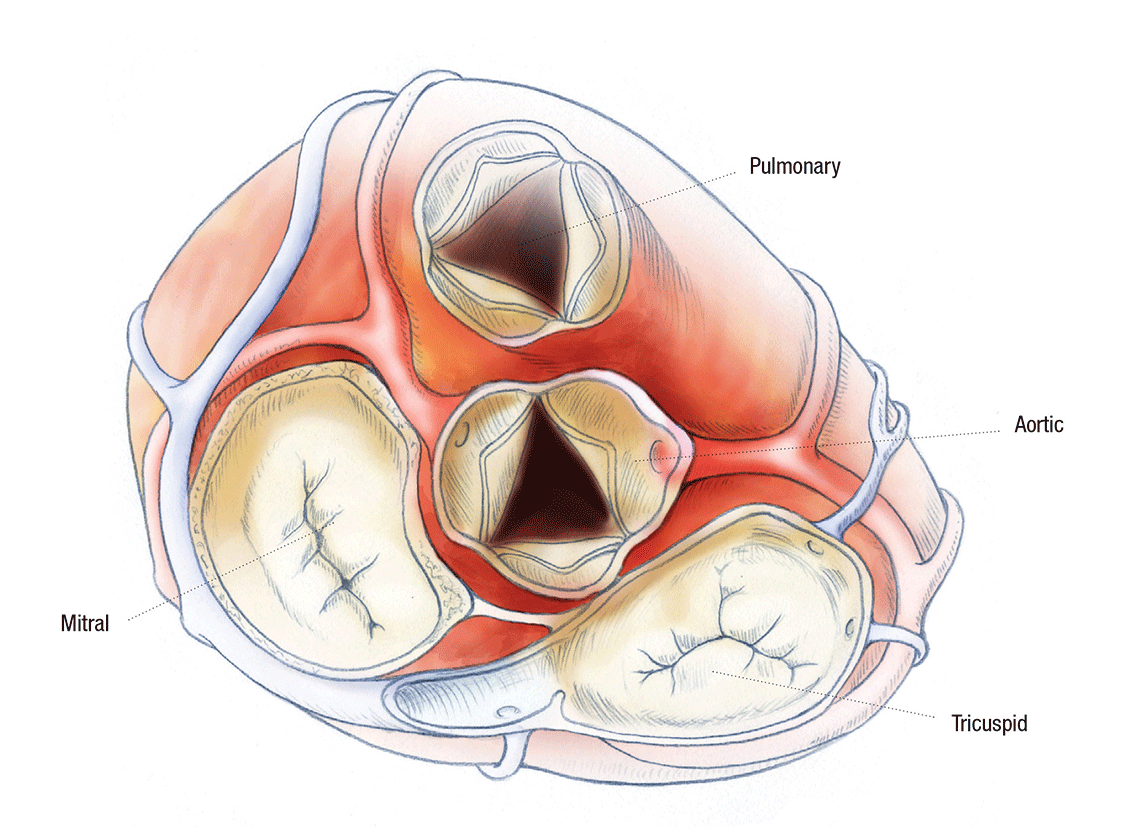
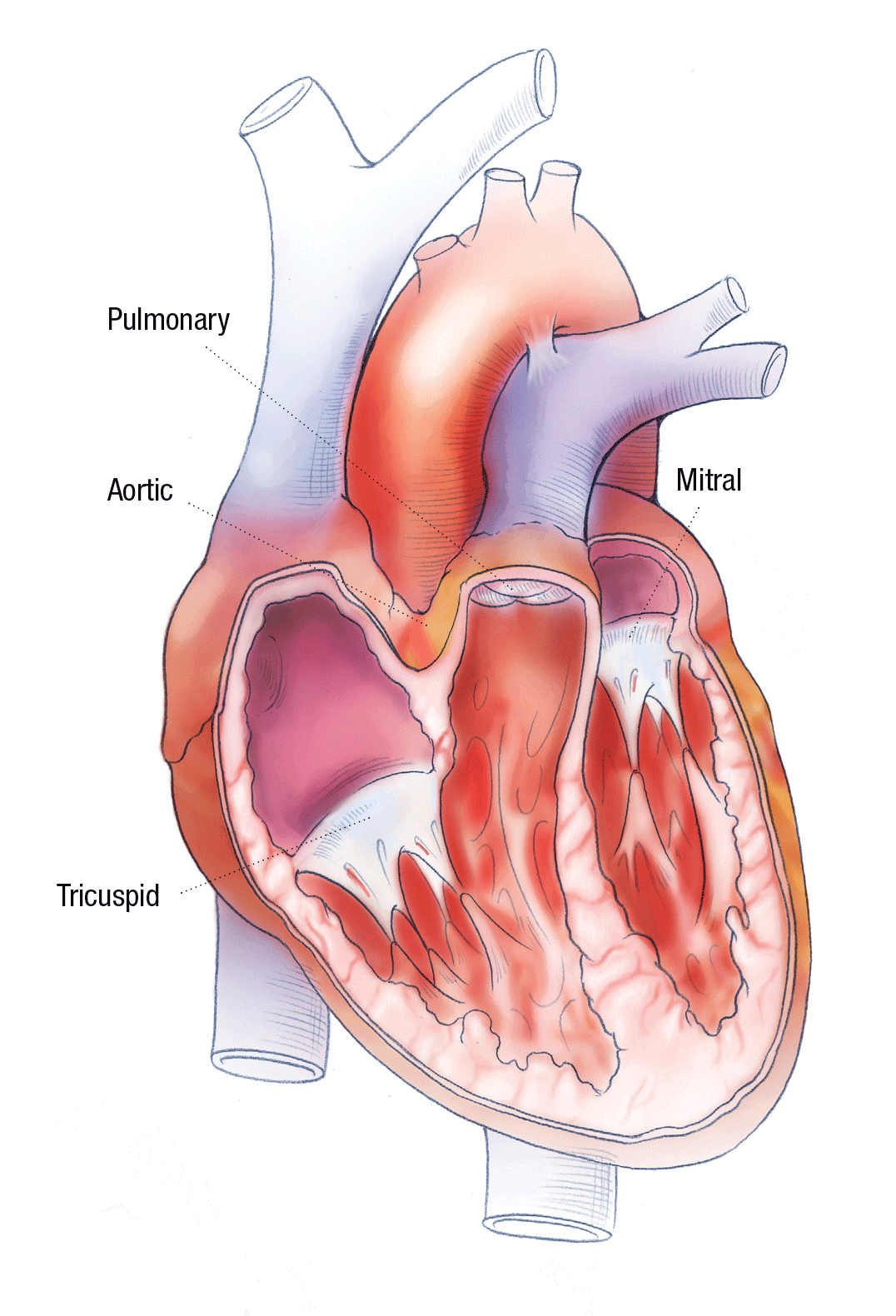
TO REPAIR OR REPLACE? THE FIRST QUESTION OF HEART VALVE SURGERY
The only options when the valve ceases to function properly are surgical attempts to repair the leaflet or annular region and to replace the valve entirely. “While repairs can sometimes delay the need for a full valve replacement, the choice ultimately becomes, how many more surgeries do we want this child to face?” says Patrick McConnell, MD, pediatric cardiothoracic surgeon in The Heart Center at Nationwide Children’s Hospital.
“Even a busy surgeon will only do pediatric heart valve repair a couple times a year,” he says. “Each child has his or her own unique heart valve problem, unlike adults whose pathology tends to remain pretty similar across patients and for whom replacement is more standardized and durable.”
When each case is a special one, and the cases are few and far between for an individual surgeon or center, developing standardized treatments is difficult.
“Repair of pediatric heart valves has not seen an epiphany like it has in adult valve surgery,” elaborates Dr. McConnell, who is also an assistant professor of Surgery at The Ohio State University College of Medicine. “Standard procedures don’t exist for pediatrics, in part because the anatomy of each pediatric case is unique and we are more often starting with too small of a valve where options are far more limited.”
And while Dr. McConnell says that repair is almost always attempted, it has to be balanced with the goal of reducing the number of times the patient and family need to see a surgeon. And so, ultimately, a patient with heart valve disease will be facing the prospect of heart valve replacement.
For pediatric patients, this involves at least one open heart surgery. For most pediatric patients, it means multiple surgeries over the course of their lives. Infants who need a heart valve replacement face three to four surgeries from infancy to adolescence to upsize the valve as the child grows. Issues with the durability of the replacement valves often result in the need for additional procedures.
Two types of prosthetic valves – mechanical and biological – are used in replacements. In children, mechanical valves are rarely used for two main reasons. First, they don’t grow and can’t be stretched or enlarged, meaning another surgery is always required. Second, the anticoagulant therapy required for mechanical valves, regardless of placement, involves higher risks and more severe side effects for children than it does for older adults.
Biological valves may be constructed from animal tissues (pig or cow) or cadaveric valve tissue and do not require anticoagulation. The drawbacks of biological valves include potential immune-incompatibility and the speed with which the leaflets degenerate.
As any parent knows, kids are hard on things. They wear out clothes and shoes and toys – and replacement heart valves.
“Children aren’t just out-growing their valves. That’s certainly a factor, but it’s not the whole story,” says Dr. McConnell. “They tend to tear through their replacement valves, and we can’t easily predict why or how or who will be most affected.”
The issue of durability of the valve in children may be related to the unique biomechanical forces the valve is exposed to in a growing child as well as the immune response of the growing child.
Heart valve researchers are now taking aim at these factors and collaborating with bioengineers to develop better solutions for congenital heart valve disease (CHVD).
“The outcomes of pediatric heart valve replacement are some of the worst results you would ever encounter in the adult world,” says Dr. McConnell. “But they are the reality in pediatrics. We cannot accept the reality that the best we can do for some of these children is six months between procedures. It’s a struggle to reduce morbidity and mortality for these kids. We have to do better.”
USING A CATHETER TO REPLACE VALVES
The invention of the Medtronic Melody transcatheter pulmonary valve replacement on a stent in 2000 – treating both stenosis and valve regurgitation without open heart surgery – created a big shift in thinking.
“Now, heart valves do not always need to be put in by a surgeon with a patient on bypass. This is huge,” explains John P. Cheatham, MD, interventional cardiologist and co-director of The Heart Center at Nationwide Children’s. “The risks associated with cardiopulmonary bypass compound with repeated open heart surgeries. But with the transcatheter approach, you can come in the morning, get a new valve and go home the next day.”
“Pediatric patients will almost always still need an open heart surgery first,” says Dr. Cheatham, who holds the George H. Dunlap Endowed Chair in Interventional Cardiology. “But if we can reduce the number of open heart procedures over the child’s lifetime by giving them a transcatheter option that is equal to or better than surgery, we’ll be making a huge improvement in morbidity and mortality.”
Nationwide Children’s, in an effort led by Dr. Cheatham, was one of five sites in the United States to test the Melody TPV. This study led to the approval by the Food and Drug Administration (FDA) in 2010 for valve replacement in pulmonary outflow tracts that have a surgically placed conduit; that is, for patients who have already had at least one pulmonary valve replacement with a conduit. This patient population accounts for approximately 20-25 percent of people who have had surgery in that area.
The Medtronic Harmony transcatheter pulmonary valve, which is now in clinical trial, was designed using the same concept as the Melody valve but to fit in patients who did not receive a surgical conduit – approximately 75 percent of the people who have had surgery in this area. Historically, patients with congenital heart disease who had narrowing around the pulmonary valve and artery had a transannular patch inserted to widen the area, and the valve was sacrificed. Because the patients appeared to do well after the surgery, even without their pulmonary valve, it was generally believed that it wasn’t absolutely necessary to replace the pulmonary valve in these cases, explains Dr. Cheatham, who is also a professor of Pediatrics and Internal Medicine at The Ohio State University College of Medicine.
Nationwide Children’s was one of three sites worldwide to test this new valve in the FDA’s first early feasibility study, led locally by Dr. Cheatham. Now, Dr. Cheatham serves as the national principal investigator for the pivotal trial.
“Now that these patients are living longer, we are learning that going along without a pulmonary valve is really not good for the long term. Patients may survive initially, but eventually, we’re seeing that the regurgitation into the pumping chamber is causing heart failure,” says Dr. Cheatham. “Adults with CHD who had this surgery in childhood are having to go back for open heart surgery to place a conduit or pulmonary valve. The Harmony is designed to be a transcatheter option for this group.”
While advances in transcatheter approaches are promising, each size of transcatheter valve and conduit requires additional engineering and clinical study before it can be made available to the public. Additionally, researchers are following patients who have had transcatheter valve replacements to understand what impact, if any, leaving the diseased valve or the worn-out replacement valve in the body has.
And durability is still a huge issue. The valves delivered by transcatheter approach are still biologic valves without growth potential and prone to degradation and calcification.
“In the future, we’d like to have a better valve to implant with the transcatheter approach,” says Dr. Cheatham. “A tissue engineered valve made from the patient’s own cells that could grow and remodel, delivered without surgery – now that would be a game changer.”
“Using a transcatheter approach to fix a fetal heart valve in utero, using a tissue engineered valve, could result in a baby that would have faced a lifetime of procedures being born with no defect and no need for additional surgery,” adds Dr. Cheatham. “This is an exciting opportunity, and I think we’re just beginning to see how fetal heart catheterizations can be used.”
DESIGNING A TISSUE ENGINEERED HEART VALVE
Tissue engineered heart valves (TEHVs) have been teetering between the bench and the bedside for the last 20 years since Christopher Breuer, MD, and Toshiharu Shinoka, MD, PhD, co-directors of the Tissue Engineering Program at Nationwide Children’s, created the first TEHV at Harvard University.
“Our first heart valve was a scaffold seeded with cells that formed a functioning valve that was alive and could grow and remodel,” says Dr. Breuer, who holds the Nationwide Endowed Chair in Surgical Research. “As we turned our focus to moving tissue engineered vascular grafts into the clinic, others carried the torch of the TEHV.”
According to Drs. Breuer and Shinoka, the investigation into TEHV follows two fundamental approaches. In the first approach, a biodegradable polymer scaffold is seeded with cells from the patients. The cells then make their own extracelluar matrix (ECM) before and after implantation. In the second approach, researchers start with an established ECM – for example a decellularized donor valve. Then cells are added in vitro or the ECM acquires cells in vivo. Neither of these approaches has resulted in reliably good clinical results, but the research is moving forward.
In June 2017, experts from around the world gathered at Nationwide Children’s to discuss the state of tissue engineered vascular grafts and valves at the International Society for Vascular Tissue Engineering Symposium. There, John Mayer Jr., MD, senior associate in cardiovascular surgery at Boston Children’s Hospital and professor of surgery at Harvard Medical School, gave a special presentation about the past, present and future of TEHV.
During his talk, he called for investigation into the mechanical and microenvironments of the cells involved in the scaffold or ECM. Dr. Mayer suggests that creating a multilayered scaffold and refining ways to test the valve before placing it in the animal model should be the next steps toward a clinically useful TEHV.
COLLABORATING WITH ENGINEERS
In a lab at the Dorothy M. Davis Heart and Lung Research Institute at The Ohio State University, associate professor of biomedical engineering and surgery Lakshmi (Prasad) Dasi, PhD, leads a team in the application of engineering principles to heart valves. At the heart of his lab is a bioreactor that can do just what Dr. Mayer suggests.
“My lab is focused on understanding the role of valve mechanics on the initiation of native valve diseases or performance of novel prosthetic valves. While we are currently focused on calcific aortic valve disease, you could use a similar system to model the conditions of any valve,” says Dr. Dasi. “The engineering side of this project studies detailed blood flow patterns through the valve, how the leaflets open and close in response to these patterns, and what mechanical signals could trigger disease processes.”
To learn about how mechanics impact disease, Dr. Dasi and his team have designed an incubator-sized bioreactor system that enables them to control and measure nearly every variable influencing the valve.
“The bioreactor system serves as a model in which we can change local aortic root anatomy, heart rate, cardiac output, systolic durations and even coronary flow rates,” says Dr. Dasi.
The applications of this tool extend far beyond testing tissue engineered valves for durability. In one project, Dr. Dasi’s team is collaborating with Dr. Cheatham, using the flow setup to test new designs for catheter-deliverable valves. From altering the ring shape to using different polymers in the stent and finally altering the leaflet materials, researchers are working to move the needle on the durability of valves.
WHAT ROLE DOES BIOMECHANICS HAVE IN CALCIFICATION?
In a new collaboration with Joy Lincoln, PhD, principal investigator in the Center for Cardiovascular Research in The Research Institute at Nationwide Children’s, Dr. Dasi and his team are aiming to use the bioreactor to study how fluid mechanics influence the onset and progression of calcific aortic valve disease (CAVD).
CAVD is the most prevalent valvular disorder in the United States. While it has long been considered an adult disease, increasing evidence suggests that the disease has its origins during embryonic development.
In the collaboration, they will use 3D printed models of patients with CAVD to characterize the flow patterns and pressures experienced by the cells. “By comparing healthy and sick patients, we’re trying to see what’s different in terms of cellular experience,” elaborates Dr. Dasi. “Then we can take living valves in the bioreactor to learn about biological signals in response to the mechanics.”
Dr. Lincoln has studied CAVD in vitro, with cell culture models and in vivo using small animal models. The next step in that process is going ex vivo, in Dr. Dasi’s lab where she and her team can work with whole pieces of tissue or whole aortic valves.
“We’re trying to explore the geometries and hemodynamic parameters that affect the valve short-term,” says Dr. Lincoln, who is also an associate professor of Pediatrics at The Ohio State University College of Medicine. “We also want to look for biomarkers or molecular indicators of early stages of calcification to prevent progression to end stage valve failure.”
Through her work in vivo and in animal models, Dr. Lincoln has teased out some of the signaling pathways involved in calcification.
By understanding the mechanisms of calcification, she hopes to find strategies or drugs to slow or eliminate the calcification process, thus providing a pharmaceutical option for the treatment of CAVD.
Dr. Lincoln is testing one such drug in animal models. The phase 1 clinical trial drug – KPT-330 – is currently being tested for cancer treatments. And based on her in vitro studies, it looks promising to treat or prevent calcification as well.
“Now we’re doing some high throughput screening to try and work out how exactly the drug is working, as well as in vivo studies to rescue mouse models of calcification with the drug,” she says.
Parallel molecular studies are also ongoing to determine the molecular mechanisms by which KPT-330 can treat CAVD.
Another avenue of research in Dr. Lincoln’s lab is studying the development of heart valves in the embryo. “There’s still so much to learn about how the valves form in utero, what signaling pathways are important, how the leaflets form,” she says. “If we can understand this, it will open new avenues of research and understanding how the leaflets repair and remodel over the course of a lifetime.”
USING GENOMICS TO UNCOVER THE ORIGINS OF HVD
In the 1990s, mouse knockout technology enabled the study of the function of genes in an animal model. In the case of cardiovascular diseases, researchers identified numerous genes, which, when knocked out or deleted in the mouse, would result in the lethality of the embryo often due to improper heart development.
“While we figured out that it was due to defective heart development, these findings did not translate into the patients we see with congenital heart disease, because they’re born alive,” explains Vidu Garg, MD, director of the Center for Cardiovascular Research at The Research Institute and a pediatric cardiologist in The Heart Center at Nationwide Children’s. “That’s what stimulated me to look at this question starting from a human genetics approach. We’ve always thought there were genetic contributors, and so that’s why we began collecting DNA samples from patients with congenital heart disease.”
Dr. Garg’s research focuses on the genetic origins of bicuspid aortic valve disease, which affects adults and children alike. “With bicuspid aortic valve disease, there’s a spectrum,” he explains. “You may have a thickened dysplastic valve that presents on day one. Others may not present until much later.”
Interestingly, bicuspid aortic valve runs in some families. “As such, it’s one of the congenital heart defects that we’ve been able to find a genetic basis for,” says Dr. Garg, who holds the Nationwide Foundation Endowed Chair in Cardiovascular Research.
The first causative gene that was discovered is NOTCH1. “We found that mutations in NOTCH1 cause bicuspid aortic valve and calcific aortic valve disease in families,” says Dr. Garg. “This finding opened the door to study how mutation of NOTCH1 can cause bicuspid aortic valve and predispose an individual to valve calcification. This has allowed for the development of mouse models with mutations in NOTCH1 that display both bicuspid aortic valve and calcification.
“Currently, we know that mutation of NOTCH1 is implicated in the defect and potentially in the development of valve calcification, but we don’t know how or why exactly. There’s a lot of controversy in this area,” says Dr. Garg. “Many researchers are focusing on the abnormal flow across the bicuspid valve. My bias as a geneticist is that the aortic valve cells are going to be abnormal, as many of the genes, including NOTCH1, expressed in development are expressed in adulthood. It’s not a far stretch to say that the cells aren’t normal, even if they may look normal, because the cells contain a gene that is mutated.”
Bicuspid aortic valve is also associated with getting an aneurysm of the ascending aorta, and researchers are currently studying if loss of NOTCH1 plays a role in this process.
“If we can understand how the genetic mutations are impacting the cellular pathways and processes to cause bicuspid aortic valve and its complications of valve calcification and aortic aneurysm, we could potentially unlock the underlying mechanisms for these associated diseases,” he explains.
As CHD care continues to improve and more people with CHD live longer and have babies, physicians and geneticists will get a clearer genetic picture of the heritable pattern of specific types of CHD. Ultimately, the goal is to allow for more personalized care so that physicians and patients can know the risks of future complications and what to look for. “By understanding the genetics and underlying mechanisms of valve disease, we hope that it will ultimately lead to new therapies,” Dr. Garg says.
Another interesting area of Dr. Garg’s research looks at environmental attributes that influence genes during development and disease. For example, in developing more accurate animal models of valve disease, he and his collaborators discovered that exposure to nitric oxide influences the phenotype in models with mutations in NOTCH1.
“We think that the interactions of genes and environmental stressors are likely key in the development of heart disease – including valve disease – and this occurs even in the growing fetus,” says Dr. Garg. “If we know that there’s a certain environmental attribute that influences an important cardiac molecular pathway, why can’t we alter that?”
Dr. Garg suggests that by more closely monitoring genetic susceptibility to environmental risk factors in pregnancies we could prevent CHD for some babies.
“But we’re still trying to identify all the genetic players,” Dr. Garg adds.
BRINGING IT ALL TOGETHER
Because of the collaborative work being done across the spectrum of HVD research, the future for treatment is likely to be multifaceted – full of innovations from many diverse areas of science and clinical care.
“Looking to the future, our goal is to find ways to prevent HVD where we can offer multiple choices for treatment – pharmaceuticals, surgery or catheterization procedures – to fix valves that are not properly developing,” says Dr. Garg. “Each of these three areas, supported and integrated by biomechanical and tissue engineering, is critical to the long term success for heart valve patients.”
References:
- Dasi LP, Grande-Allen J, Kunzelman K, Kuhl E. The pursuit of engineering the ideal heart valve replacement or repair: A special issue of the Annals of Biomedical Engineering. Annals of Biomedical Engineering. 2017 Feb;45(2):307-309.
- Kerstjens-Frederikse WS, van de Laar IM, Vos YJ, Verhagen JM, Berger RM, Lichtenblet KD, Klein Wassink-Ruiter JS, van der Zwagg PA, du Marchie Sarvaas GJ, Bergman KA, Bilardo CM, Roos-Hesselink JW, Janssen JH, Frohn-Mulder IM, van Spaendonck-Zwarts KY, van Melle JP, Hofstra RM, Wessels MW. Cardiovascular malformations caused by NOCH1 muctations do not keep left: data on 428 probands with left-sided CHD and their families. Genetic Medicine. 2016 Sep;18(9):914-923.
- Koenig SN, Bosse K, Majumdar U, Bonachea EM, Radtke F, Garg V. Endothelial Notch 1 is required for proper development of the semilunar valves and cardiac outflow tract. Journal of the American Heart Association. 2016;5:e003075.
- Lincoln J, Garg V. Etiology of valvular heart disease – genetic and developmental origins. Circulation Journal. 2014 Aug;78:1801-1807.
- Mayer JE. Making progress toward a tissue engineered heart valve. The Journal of Thoracic and Cardiovascular Surgery. 2016;152(4);1165-1166.
- Shinoka T, Miyachi H. Current status of tissue engineering heart valve. World Journal for Pediatric and Congenital Heart Surgery. 2016;7(6):677-684.
Image credits: Nationwide Children’s
About the author
Abbie (Roth) Miller, MWC, is a passionate communicator of science. As the manager, medical and science content, at Nationwide Children’s Hospital, she shares stories about innovative research and discovery with audiences ranging from parents to preeminent researchers and leaders. Before coming to Nationwide Children’s, Abbie used her communication skills to engage audiences with a wide variety of science topics. She is a Medical Writer Certified®, credentialed by the American Medical Writers Association.
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
- Posted In:
- Features





