Growing Tissue to Help Children With Short Bowel Syndrome
Growing Tissue to Help Children With Short Bowel Syndrome https://pediatricsnationwide.org/wp-content/uploads/2021/03/050316BS019G-1-besner-header-1024x575.gif 1024 575 Kevin Mayhood Kevin Mayhood https://secure.gravatar.com/avatar/bd57a8b155725b653da0c499ae1bf402?s=96&d=mm&r=g- March 08, 2018
- Kevin Mayhood
Research using a small animal model shows that it matters where in the body the cultured intestine is grown.
Researchers at Nationwide Children’s Hospital are hoping to use short bowel syndrome patients’ own cells to grow extra tissue needed for their small intestine to function properly.
Using rat models, the team of physician-scientists found that where they incubate segments of small intestine inside the abdomen can produce tissue with different characteristics.
“Short bowel syndrome (SBS) usually occurs when a surgeon is faced with the need to remove long lengths of diseased small bowel in the operating room in order to save a patient’s life,” says Gail Besner, MD, chief of General Pediatric Surgery at Nationwide Children’s and senior author of the research, published in Tissue Engineering Part A and Journal of Surgical Research. “The patient cannot absorb enough nutrients post-operatively because there is not enough length of remaining small intestine.”
SBS can be deadly and costly. The mortality rate for children with the syndrome is 15 to 25 percent due to complications related to the use of total parenteral nutrition, published research shows. This intravenous feeding method costs upwards of $100,000 per patient annually.
Moreover, the five-year survival rate for children receiving an intestinal transplant is also low, largely because the immunosuppression required to prevent rejection makes transplant recipients susceptible to life-threatening infections.
“The concept behind our research is to produce tissue engineered tissue made from the patient’s own cells so that it won’t be rejected by the patient’s immune system,” Dr. Besner says. “We take a tiny part of their remaining intestine, and culture it and expand it in the lab.”
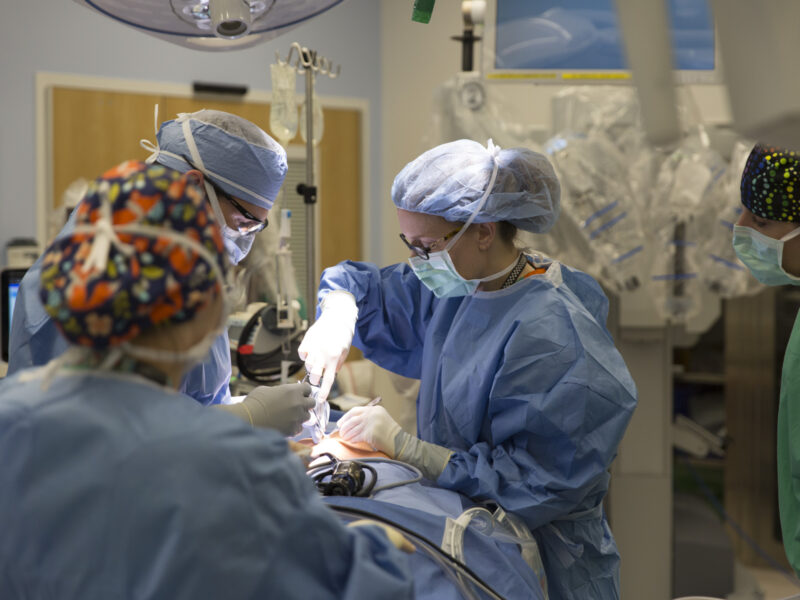
The researchers start with a tiny biopsy of small bowel and use it to grow minuscule portions of intestine called organoids, which contain intestinal stem cells surrounded by myofibroblasts, fibroblasts and smooth-muscle cells. They expand these organoids in culture several hundred-fold and then seed them onto tubular-shaped polyglycolic acid scaffolds.
“Under optimal conditions, the cells proliferate and after several weeks of implantation in the abdominal cavity, tissue engineered intestine is produced,” Dr. Besner says.
The researchers grew seeded scaffolds in five intra-abdominal locations: wrapped in intestinal mesentery (the tissue that contains the blood vessels supplying blood to the intestine), wrapped in omentum (a vascular membrane attached to the stomach), wrapped in uterine horn membrane, attached to the abdominal wall or inserted in the subcutaneous space.
They found that seeded scaffolds wrapped in any of the three membranes, which are highly vascularized, produced the greatest volume and highest-quality tissue-engineered intestine after four weeks.
Immunofluorescent staining demonstrated the presence of components of the enteric nervous system as well as blood vessels. Those grown in the vascularized membranes had significantly greater blood vessel density than the others.
The challenge remains to fully innervate the engineered tissue and to have the right mix of cell types present so that after surgically attaching engineered segments to existing intestine the grafted segments will peristalse and absorb nutrients, Dr. Besner says.
She and her team recently received new National Institutes of Health funding and are preparing to test and further develop the technology in a large animal model.
References:
- Liu Y, Cromeens BP, Wang Y, Fisher K, Johnson J, Chakroff J, Besner GE. Comparison of different in vivo incubation sites to produce tissue-engineered small intestine. Tissue Engineering Part A. 2018 Jan 31. [Epub ahead of print]
- Cromeens B, Liu Y, Stathopoulos J, Wang Y, Johnson J, Besner G. Production of tissue-engineered intestine from expanded enteroids. Journal of Surgical Research. 2016 Jul;204(1):164-75.
Photo credit: Nationwide Children’s Hospital
About the author
-
Kevin Mayhoodhttps://pediatricsnationwide.org/author/kevin-mayhood/
-
Kevin Mayhoodhttps://pediatricsnationwide.org/author/kevin-mayhood/April 25, 2015
-
Kevin Mayhoodhttps://pediatricsnationwide.org/author/kevin-mayhood/April 25, 2015
-
Kevin Mayhoodhttps://pediatricsnationwide.org/author/kevin-mayhood/April 25, 2015
- Posted In:
- In Brief