Optimizing the Body’s Natural Cancer Killers
Optimizing the Body’s Natural Cancer Killers https://pediatricsnationwide.org/wp-content/uploads/2022/04/NK-Cell-Cover-Art-web-crop-1024x649.jpg 1024 649 Katie Brind'Amour, PhD, MS, CHES Katie Brind'Amour, PhD, MS, CHES https://pediatricsnationwide.org/wp-content/uploads/2021/03/Katie-B-portrait.gif- April 20, 2022
- Katie Brind'Amour, PhD, MS, CHES
Recent advances in the expansion and production of natural killer cells offers pediatric patients new hope for remission after high-risk cancer diagnoses.
Natural killer (NK) cells are the innate immune system’s first line of defense for viral infections. Although these white blood cells don’t have the antigen-specific “memory” that characterizes T cells or the antibody-producing capacity characteristic of B cells, they act faster than T or B cells because they do not require priming — prior activation or exposure to a pathogen or tumor cell — in order to recognize and attack problematic cells.
Instead, NK cells operate on constant patrol, bumping into other cells and checking for signs of cancer, infection or metabolic stress via receptors on their surface. Certain signals and molecules from potentially dangerous cells activate NK cell receptors and trigger a full-scale assault: the NK cell releases toxic granules that infiltrate the target cell and cause cell death.
In healthy individuals, NK cells — and the army of immune cells they recruit for assistance — keep most infections and potential tumors at bay. But there are important limitations.
Sometimes viral replication is too fast for the NK cells to manage alone, and an infection takes root until the rest of the system kicks into gear, or a tumor may develop mechanisms of hiding from immune cells. While medicine can help with some of these circumstances, in others, we have few treatment options at our disposal to achieve what our bodies cannot.
Some treatments for high-risk pediatric cancers, such as acute myeloid leukemia (AML), cause damage to the innate immune system; these include chemotherapy and radiation, which kill cells of the innate immune system and temporarily stop NK cells from continuing their valuable work. Furthermore, a potentially curative treatment for leukemia patients, hematopoietic stem cell transplant (HSCT), requires immune suppression for several months.
Outcomes for these already high-risk patients take a nose-dive when post-transplant infections hit before the immune system has recovered (most often in the first 6 months after the procedure). Without a functional stock of NK cells circulating to fight off invaders, many patients fall prey to common illnesses. Worse still, the lack of NK cells results in an immune system unable to detect early reemergence of leukemia cells, resulting in a cancer relapse.
To address the immune suppression caused by these cancer therapies, many scientists have been methodically cultivating the concept of lab-made or cultured immune cells. From NK cells to CAR T cells (chimeric antigen receptor T cells, which are genetically altered to fight specific cancer antigens), innovations in immune cell research are poised to revolutionize the field of immunotherapy.
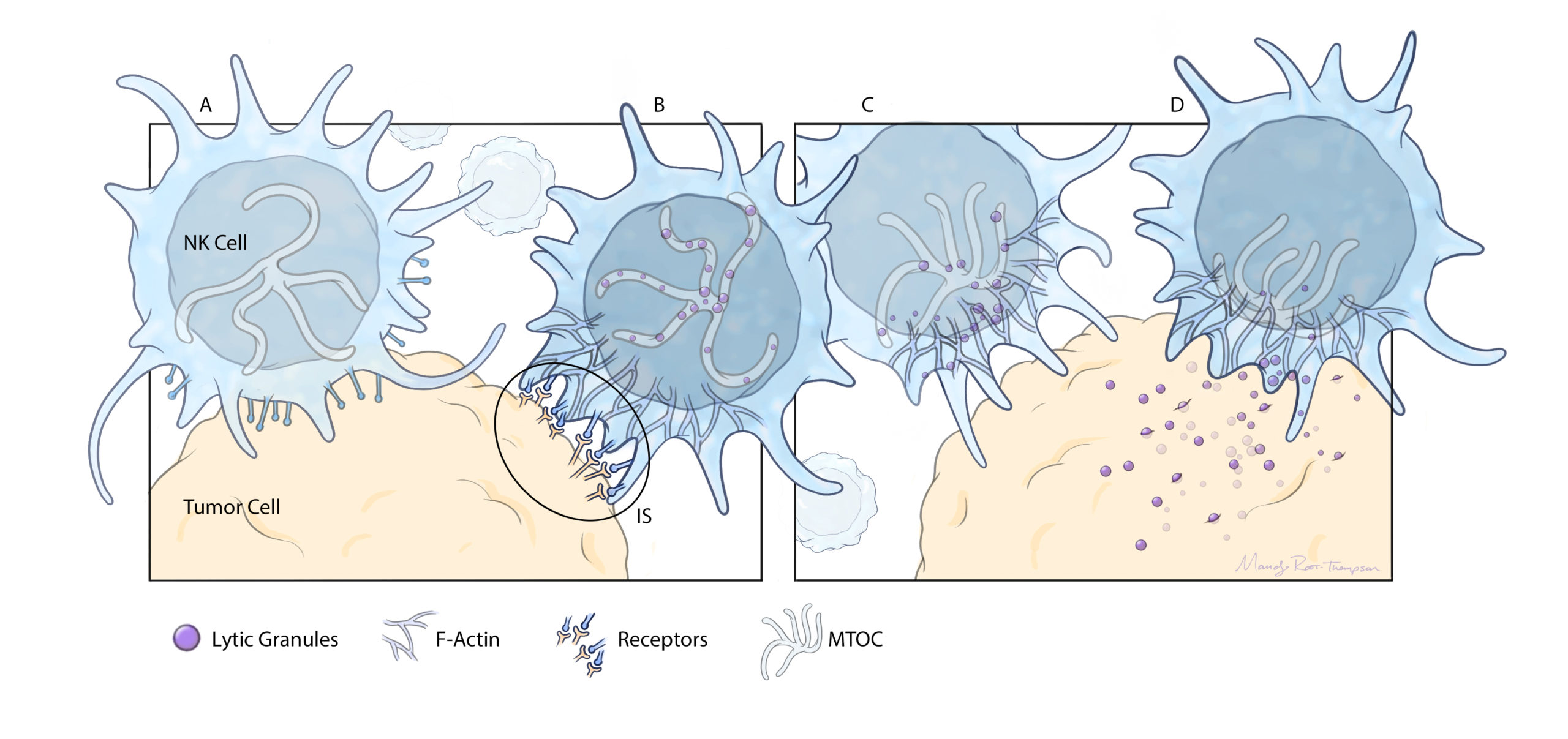
HOW THE NK CELLS WORK
A: NK cells are in constant circulation in the bloodstream, bumping into other cells in an attempt to detect invaders, such as viral or tumor cells.
B: When they collide with invaders, receptors on the NK cell surface identify dangerous proteins or antigens produced by the foreign cells.
C: These antigens trigger an attack from the NK cell. It activates F-actin, a group of bundled, polarized polymers that stabilize the NK cell’s attachment to the foreign cell’s membrane.
D: The NK cell releases lytic granules from its microtubule-organizing center (MTOC). These killer granules pass into the foreign cell and
trigger cell death.
E: Natural killer cells can be extracted from donors, expanded in the lab, and infused directly into patients.
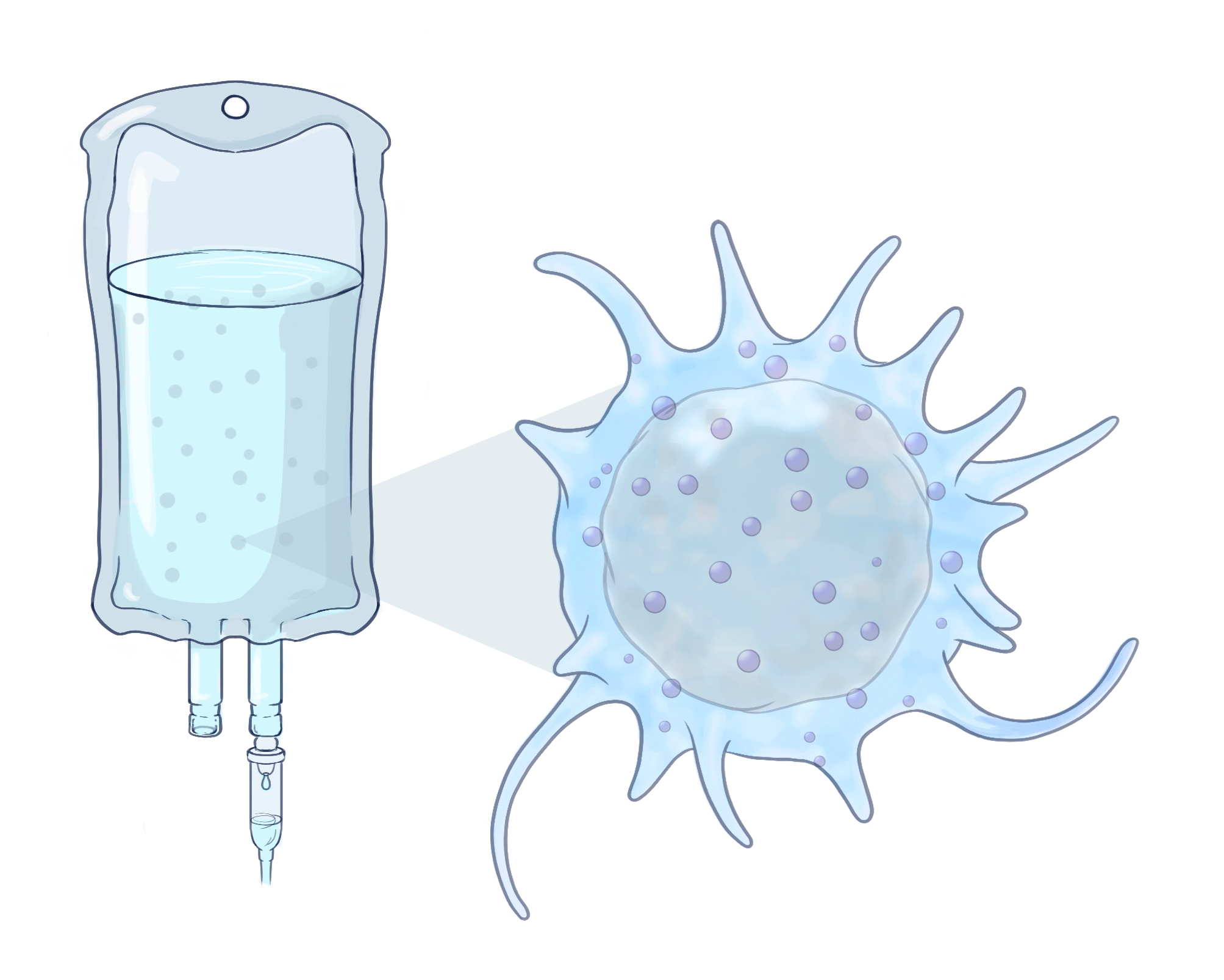
MAKING MAGIC FROM DONOR NK CELLS
Unfortunately, NK cells have proven difficult to select from blood donations, and the relatively small quantities obtained naturally may not be enough for effective therapies. Furthermore, there appears to be natural variation in the quality of our NK cells, and they may not last long enough for a single infusion to do much good. Multiple doses may be required to maximize protective potential after chemotherapy or stem cell transplant. Traditionally, to make this work, a donor would have to repeatedly give blood or undergo a longer apheresis procedure to collect enough starting material.
“I have been studying the biology of NK cells in the lab and their clinical use as immunotherapy for well over a decade and have been really amazed by the sophisticated power of these immune cells,” says Monica Thakar, MD, director of Bone Marrow Transplantation Inpatient Services at Seattle Children’s Hospital and an associate professor of pediatrics at Fred Hutchinson Cancer Research Center and University of Washington.
Her two initial NK cell clinical trials in children with high-risk cancers infused haploidentical, or “half-matched,” NK cells from family members to prevent post-HSCT cancer relapse. NK cells were isolated from the donors’ peripheral blood obtained via apheresis, a lengthy and technically complicated procedure. The purified NK cells were then directly infused into patients the same day of collection, as a fresh cell therapy product.
“I realized that maybe we needed a lot more NK cells to go in, or that we needed to do something to the cells so that they persisted or were more activated than just our single fresh infusion,” says Dr. Thakar. “We were making an impact, but we kept hitting limitations. That’s where Dean and I connected.”
Dean Lee, MD, PhD, a bone marrow transplant specialist and director of the Cellular Therapy and Cancer Immunology Program at Nationwide Children’s Hospital, joined the world of NK research by chance. Early in his career, while working on methods to expand T cells using genetically engineered feeder cells, he observed that about 10-20% of the time, dramatic NK cell overgrowth occurred instead. Encouraged by his advisor at the time, he investigated whether he could reliably direct the growth of NK cells from donor blood using the feeder cells and various combinations of cytokines and cell stimulants.
THE NEXT PHASE IN NK CELL RESEARCH
“The one drawback with the current trial is that Dr. Lee is manufacturing these infusions from donor blood, so it still takes a couple of weeks to expand the cells,” says Dr. Thakar, who is developing her own NK cell program at Fred Hutchinson Cancer Research Center. “It would be so much easier to have an ‘off-the-shelf ’ product that is ready to thaw and infuse on demand.”
Dr. Lee agrees. He learned from his AML studies in adults that waiting for family donors and patient-by-patient cell expansion can take too long, allowing some patients to fall ill by the time the cells are ready to infuse.
That’s why he’s been working to tease out what it is about some donors’ cells that makes for a highly expandable, potent, efficient response to cancer and infection, so that he can identify “super donors” from cell banks and generate large quantities of NK cells using their donations. These cells can then be cryopreserved, banked and kept available for patients whenever needed. This could make or break treatment success in very ill patients who require an urgent immune boost. It also enables the use of NK cells for post-chemotherapy infusions and post-transplant infusions without any added burden on a family donor.
“For patients with AML or other rapidly proliferating diseases, one challenge in immune cell therapies is that, because they require autologous products, there is a lag in time while we manage cell collection and then cell expansion ex vivo,” says Dr. Bonifant. “The opportunity to have cells immediately available from either the transplant or other allogeneic donor would decrease costs. More importantly, it would decrease the time between identifying a patient in need and getting them the cell therapy product. It’s like a dream for this population.”
Dr. Lee’s work toward this aim is almost ready for prime time. His team has already identified a handful of optimal donors that have the “best” anti-cancer NK phenotype from a national registry and begun the process of expanding cells; multiple pediatric post-chemotherapy infusion trials using the universal NK cell donor product will launch this year.
AML IS JUST THE BEGINNING
AML was an excellent first target for pediatric NK cell-focused research, but the utility of NK cells may extend far beyond AML.
“There is potential for NK cell therapy in every kind of cancer,” says Dr. Lee. “We haven’t found any cancer yet that is uniformly resistant to NK cells — some patients may have resistant tumors, but there’s promise in any cancer type. Moving forward, we can test whether giving large numbers of high-functioning NK cells will do something to whatever cancer you want to try.”
Dr. Lee’s team has already done studies in brain tumors and neuroblastoma, and together with The Ohio State University, they are set to open new studies for sarcomas, melanoma, breast cancer, T-cell lymphomas and skin lymphoma, and additional studies for AML, neuroblastoma and brain tumors. And the EXCEL study investigators feel this is just the beginning.
“In a perfect world, we could think about injecting them directly into solid tumors to get past the physical barriers impeding their infiltration, or maybe combining NK cells with different classes of drugs to overcome immunologic barriers of the tumor microenvironment,” says Dr. Thakar. NK cell therapies developed using other donor and expansion modalities have already been tried in high-risk solid tumors with modest success. “We have lots of work to do to see what’s possible, and we will be working hard to establish safety and explore other avenues for pediatric cancer, including treating active relapse.”
These experts are not the only ones to recognize the potential of this technology. NK cell therapies utilizing Dr. Lee’s expansion method are currently under study by a handful of pharmaceutical companies in adults with leukemias, neuroblastomas and more. In addition, investigators around the world are working to generate NK cells from induced pluripotent stem cells, exploring alternative ways to expand and infuse them or, like Drs. Lee, Thakar, Bonifant and their colleagues, exploring ways to alter NK cells to target specific cancers by creating CAR-NK cells.
“Can we overcome suppressive factors in the tumor microenvironment and redirect NK cells to identify specific tumors?” Dr. Lee asks. “Can we combine NK cells with antibodies or other immune-modulating drugs to improve survival and function? That’s what is coming in the next NK innovation wave.”
To that end, the CRISPR/Gene Editing Core staff at Nationwide Children’s and The Ohio State University, led by Meisam Naeimi Kararoudi, DVM, PhD, has been working on CAR-NK cells for the past five years. Their work with Margaret Lamb, MD, a pediatric oncologist and HSCT specialist at Nationwide Children’s and lead investigator for the upcoming universal NK cell AML trial, has allowed the team to develop new CAR-NK cell study protocols as well.
“We’re really excited about continuing our work with NK modification to see what can help children the most,” says Dr. Lamb.
The NK team at Nationwide Children’s has plans for growth for the cell production capacities both of donor-specific NK and CAR-NK cell lines and universal cell batches. The GMP facility will be able to support
numerous multi-center trials simultaneously, especially if the universal donor concept proves successful.
“We hope to have the first CAR-NK cell clinical trials by the end of this year,” Dr. Lamb says, “with many more to come.”
This article appears in the 2022 Spring/Summer print issue. Download the full issue.
References:
- Ciurea SO, Kongtim P, Soebbing D, Trikha P, Behbehani G, Rondon G, Olson A, Bashir Q, Gulbis AM, Indreshpal K, Rezvani K, Shpall EJ, Bassett R, Cao K, Martin AS, Devine S, Horowitz M, Pasquini M, Lee DA, Champlin RE. Decrease post-transplant relapse using donor-derived expanded
NK-cells. Leukemia. 2022 Jan;36(1):155-164. - Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, Singh H, Hurton L, Maiti SN, Huls MH, Champlin RE, Cooper LJN, Lee DA. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7(1):e30264.
- Christodoulou I, Rahnama R, Ravich JW, Seo J, Zolov SN, Marple AN, Markovitz DM, Bonifant CL. Glycoprotein targeted CAR-NK cells for the treatment of SARS-CoV-2 infection. Frontiers in Immunology. 2021 Dec 23;12:763460.
- Khatua S, Cooper LJN, Sandberg DI, Ketonen L, Johnson JM, Rytting ME, Liu DD, Meador H, Trikha P, Nakkula RJ, Behbehani GK, Ragoonanan D, Gupta S, Kotrotsou A, Idris T, Shpall EJ, Rezvani K, Colen R, Zaky W, Lee DA, Gopalakrishnan V. Phase I study of intraventricular infusions of autologous ex vivo expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro Oncology. 2020;22(8):1214-25.
- Silla L, Valim V, Pezzi A, da Silva M, Wilke I, Nobrega J, Vargas A, Amorin B, Correa B, Zambonato B, Scherer F, Merzoni J, Sekine L, Huls H, Cooper LJ, Paz A, Lee DA. Adoptive immunotherapy with double-bright (CD56bright/CD16bright) expanded natural killer cells in patients with relapsed or refractory acute myeloid leukaemia: a proof-of-concept study. British Journal of Haematology. 2021;195(5):710-721.
- Yang C, Siebert JR, Burns R, Gerbec ZJ, Bonacci B, Rymaszewski A, Rau M, Riese MJ, Rao S, Carlson KS, Routes JM, Verbsky JW, Thakar MS, Malarkannan S. Heterogeneity of human bone marrow and blood natural killer cells defined by single-cell transcriptome. Nature Communications. 2019 Sep 2;10(1):3931.
Photo credits: Nationwide Children’s (Drs. Lee, Rangarajan, and Lamb); Drs. Bonifant and Thakar photos used with permission.
Illustration credit: Mandy Root-Thompson for Nationwide Children’s
About the author
Katherine (Katie) Brind’Amour is a freelance medical and health science writer based in Pennsylvania. She has written about nearly every therapeutic area for patients, doctors and the general public. Dr. Brind’Amour specializes in health literacy and patient education. She completed her BS and MS degrees in Biology at Arizona State University and her PhD in Health Services Management and Policy at The Ohio State University. She is a Certified Health Education Specialist and is interested in health promotion via health programs and the communication of medical information.
- Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
- Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
- Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
- Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 28, 2014