The Transfusion Evolution
The Transfusion Evolution https://pediatricsnationwide.org/wp-content/uploads/2015/10/bloodbag_illustration.jpg 576 367 Abbie Miller Abbie Miller https://pediatricsnationwide.org/wp-content/uploads/2023/05/051023BT016-Abbie-Crop.jpg- October 23, 2015
- Abbie Miller

The first successful open heart surgery with cardiopulmonary bypass was performed in 1953, but it wasn’t until the 1970s that these surgeries began to have high success rates — due in large part to the availability of fresh whole blood transfusions.
However, fresh whole blood is difficult to attain. In response, blood component transfusions became a more popular solution. As techniques for administering blood components improved, physicians reaped the added benefit of giving patients only the components they needed.
Now, the evolution of cardiothoracic surgery and transfusion medicine continues with advances in surgery, anesthesiology and perfusion, ushering in a new era in which whole and component blood transfusions may become a thing of the past.
Undoubtedly, blood and blood product transfusions save lives. But while blood transfusions are much safer now, inherent risks are still associated with using donated blood products, including allergic reaction, infection and increased edema and inflammation.
Furthermore, the availability of allogeneic blood products is not guaranteed. According to the American Red Cross, more than 41,000 blood donations are needed every day and 300 million blood components are transfused each year in the United States. Conventional wisdom emphasizes limiting the use of blood products.
However, how to safely achieve that goal during pediatric cardiac surgery is still up for debate.
Chief of Cardiothoracic Surgery Mark Galantowicz, MD, and his team in The Heart Center at Nationwide Children’s Hospital are challenging the culture of transfusion with new techniques and aggressive strategies of blood conservation for all their patients.
PHILOSOPHY OF CELL SALVAGE
One technique used with great success at The Heart Center is cell salvage — saving blood that is shed or removed from the body so that it can be reintroduced to the patient. And it’s more than a technique, it’s a philosophy. “We look for places to conserve the patient’s own blood inside and outside the operating room,” says Dr. Galantowicz. “For example, when you draw a blood sample from an IV, some blood diluted with saline or medicine is extracted before the clean sample is drawn. Usually, the diluted blood is just thrown away, but we give it back to the patient. It just takes a little time and effort to make this change.”
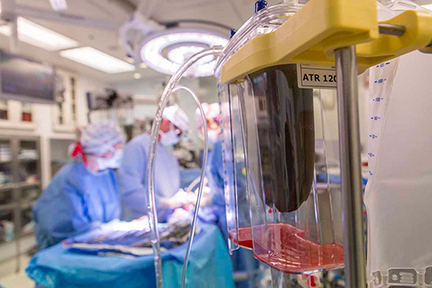
The cell saver cleans blood collected during the procedure before it is returned to the patient.
The same is true for blood that is left in the bypass circuit once surgery is complete. Many institutions dispose of this blood without attempting to give it back to the patients. However, with the use of a cell saver machine, this blood can be safely cleaned and given as an autologous transfusion.
Central to the cell salvage philosophy is the intraoperative use of a cell saver machine to collect, clean and return shed blood back to the patient. The blood collected can be given back continuously or saved for post-operative autologous transfusion.
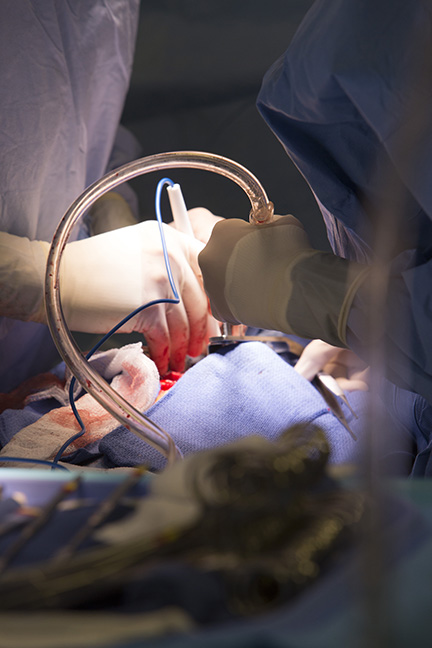
Nurses carefully suction blood during surgery. This blood is cycled through the cell saver and returned to the patient.
“Some people argue that with intraoperative cell salvage, too little blood can be collected during pediatric surgery to make it worthwhile,” says Ashley Hodge, MBA, CCP, FPP, cardiothoracic surgery quality and safety officer and perfusionist with The Heart Center. “Our nurses are vigilant about collecting blood that is lost — besides using suction, they rinse and wring out sponges and gauze pads.”
Another misconception about cell salvage relates to the safety and functionality of the red cells collected via intraoperative cell salvage. “Historically, it was believed that you couldn’t safely capture shed blood and that the red cells wouldn’t work properly when readministered. But this has been shown to be incorrect,” says Dr. Galantowicz, who is co-director of The Heart Center.
The cell saver machine is also used to wash donor blood when a transfusion is needed. “Donor blood is high in glucose, high in potassium and high in lactate. All of these things can have negative effects on the patient, including arrhythmias, kidney problems, prolonged vent times and infections,” notes Joseph Timpa, CCP, FPP, chief perfusion manager and ECMO co-coordinator at Children’s of Alabama. “Washing donor blood with a cell saver helps prevent these conditions.”
According to Dr. Galantowicz, the initial cost of the cell saver machine and the disposable component that is replaced for each surgery is well worth it. “When you compare it to the cost of donor blood and the benefit to the patient and the shortened length of stay, it pays for itself,” Dr. Galantowicz says.
HONING IN ON HEMODILUTION
Hemodilution — diluting red cell concentration in the blood — is one of the major factors to be managed during cardiopulmonary bypass, according to Dr. Galantowicz, who also is professor of Surgery at The Ohio State University College of Medicine. Several strategies to limit hemodilution are required for a surgical course free of allogenic blood products. “It’s a combination of a lot of small changes that work together to make a really big difference in the outcome,” he explains.
By reducing the size of the cardiopulmonary bypass (CPB) circuit, a smaller volume of blood is in the circuit at any given time, leaving more blood in the patient. As an added benefit, miniaturized circuits decrease inflammation that results from the blood cells reacting to the foreign material of the CPB circuit.
“We use many different circuit sizes for cardiopulmonary bypass to more closely match the circuit size to the body weight of the patient,” explains Dr. Galantowicz.
Additional whole-team strategies to limit hemodilution and reduce the need for donor blood products are applied before, during and after bypass:
- Before bypass is initiated, retrograde autologous prime and venous antegrade prime (RAP and VAP) procedures are attempted with each patient. These procedures involve displacing the crystalloid in the CPB circuit by back-bleeding the patient’s own blood into the circuit.
- During bypass, hemofiltration and zero-balance ultrafiltration (ZBUF) extract various inflammatory markers.
- After bypass, modified ultrafiltration (MUF) is used for hemoconcentration and removal of inflammatory progenitors.
In addition, vacuum-assisted venous draining (VAVD) is a strategy that Timpa and his team at Children’s Hospital of Alabama use to aid in blood conservation. “Without VAVD, drainage is dependent on gravity and a height differential from the patient to the cardiotomy reservoir,” explains Timpa. “By using VAVD, the perfusionist can decrease the tubing length even more, which translates to reduced prime volume and hemodilution.” VAVD also enables surgeons to use smaller cannulae without compromising flow.
ANH RESULTS IN FEWER TRANSFUSIONS
One of the most innovative strategies that The Heart Center team utilizes is acute normovolemic hemodilution (ANH), a process in which a percentage of the total blood volume is withdrawn prior to bypass and returned to the patient after heparin reversal. In a research study published earlier this year, this process was associated with a decreased number of transfusions.
According to Hodge, ANH is attempted in about 90 percent of heart surgeries at Nationwide Children’s. “Our process of ANH is novel,” she says. “Many people are surprised that we are able to take so much volume — up to 20 percent — at the start.”
“Our practice of ANH is unique in that we minimize crystalloid replacement during phlebotomy and use end-organ perfusion to guide fluid replacement,” Dr. Galantowicz explains. “Additionally, the blood collected during ANH has more intact platelets and fewer inflammatory markers because it does not circulate through the CPB circuit. This strengthens the patient’s ability to heal.”
“ANH is really good at minimizing blood exposure,” says Timpa.
In traditional practices, all that extra volume from crystalloids leads to worsening edema after surgery, but the difference in post-surgical results for The Heart Center’s strategy is immediately evident. “Our patients aren’t as puffy when they come out of surgery. They look like the same kid that came in to the operating room,” explains Hodge. “We are even able to extubate most patients immediately after surgery in the OR — that’s unheard of in most institutions.”
HOW LOW CAN YOU GO?
Determining the lowest acceptable hematocrit — the amount of hemoglobin in the blood — remains a complex challenge. “There is this ‘religion’ of finding the perfect hematocrit for bypass that will give you the best outcomes every time,” says Timpa. “But the research regarding the hematocrit needed for successful heart surgeries is confirming that lower numbers than previously thought are acceptable.”
The role of the hemoglobin is to deliver oxygen to the cells, tissues and organs of the body. And the amount of hematocrit that is adequate is completely dependent on oxygen demand, explains Dr. Galantowicz. Oxygen demand is variable: it depends on the tissue type, cardiac output, body temperature and activity level.
But most surgeons and families want a concrete number.
“There is no number,” Dr. Galantowicz emphasizes. “An absolute number would be based on the partial pressure of oxygen needed to move the oxygen from the hemoglobin in the blood to the mitochondria in the organs’ cells, but this number is also fluid, so it’s debated.”
According to Dr. Galantowicz, the best way to determine if the organs are getting enough oxygen is to monitor them. “We use blood tests to monitor lactate and blood gases. We monitor the brain, kidneys, liver, heart and total body perfusion during and after surgery,” he says. When oxygen delivery is in danger of becoming inadequate, the team addresses cardiac output and oxygen demand, and, if needed, transfuses red cells to boost hematocrit.
A CULTURE OF CONSERVATION
As with the conservation of any resource, blood conservation requires education, advanced technologies, innovation and behavioral change.
The first step is awareness. “Blood exposure is a risk that people don’t pay attention to as often as they should when faced with surgery,” says Timpa. “We need to work to find better ways to reduce blood exposure where we can, and educate physicians and families about the risks, benefits and options when it comes to blood conservation.”
The movement is slowly gaining traction. With innovations in blood conservation, new research into blood substitutes and education about these topics, surgical and transfusion medicine continues to mature. “In the relatively short history of open heart surgery with cardiopulmonary bypass, we’ve seen great advances,” says Hodge. “Aggressive yet safe blood conservation will be central to moving the field forward.”
“We are evolving a fuller understanding of the pros and cons of transfusion,” Dr. Galantowicz says. “Blood is life-giving, but at a cost. We need to continue to work to capture more of the benefits and reduce the risks.”
BLOOD CONSERVATION AND RELIGION
Advocating “bloodless surgery” on religious grounds has been a significant factor leading to the current state of blood conservation. Many of the innovations used for blood conservation were driven by surgeons working to meet the needs of patients and families who are Jehovah’s Witnesses.
These techniques may often be overlooked and disregarded, though, as simply putting children in harm’s way to accommodate certain belief systems.
“Many families choose Nationwide Children’s Hospital because of our history with aggressive blood conservation,” Dr. Galantowicz says. “However, the procedures and limits used for children of Jehovah’s Witnesses families are no more aggressive than those used for every other child.”
When a child needs blood products, they get blood products.
References:
- Naguib AN, Winch PD, Tobias JD, Simsic J, Hersey D, Nicol K, Preston T, Gomez D, McConnell P, Galantowicz M. A single-center strategy to minimize blood transfusion in neonates and children undergoing cardiac surgery. Pediatric Anesthesia. 2015 May, 25(5):477-486.
- Hodge AB, Preston TJ, Fitch JA, Harrison SK, Hersey DK, Nicol KK, Naguib AN, McConnell PI, Galantowicz M. Quality improvement methodologies increase autologous blood product administration. Journal of ExtraCorporeal Technology. 2014 Mar;46(1):45-52.
- American Red Cross. www.redcross.org/blood Accessed July 24, 2015.
Image credits: Nationwide Children’s
About the author
Abbie (Roth) Miller, MWC, is a passionate communicator of science. As the manager, medical and science content, at Nationwide Children’s Hospital, she shares stories about innovative research and discovery with audiences ranging from parents to preeminent researchers and leaders. Before coming to Nationwide Children’s, Abbie used her communication skills to engage audiences with a wide variety of science topics. She is a Medical Writer Certified®, credentialed by the American Medical Writers Association.
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
- Post Tags:
- Cardiothoracic Surgery
- The Heart Center
- Posted In:
- Features