Tissue Engineered Trachea: State of the Research
Tissue Engineered Trachea: State of the Research https://pediatricsnationwide.org/wp-content/themes/corpus/images/empty/thumbnail.jpg 150 150 Lauren Dembeck Lauren Dembeck https://pediatricsnationwide.org/wp-content/uploads/2021/03/Dembeck_headshot.gif- October 13, 2023
- Lauren Dembeck
The promise of tissue-engineered trachea grafts is moving closer to the clinic, as recent preclinical studies have shown successful implantation and neovascularization.
Breathing is an essential biological function that provides our bodies with the oxygen necessary for survival. However, most of us rarely think about the biological structures that make it possible. The trachea, commonly called the windpipe, is an indispensable part of the respiratory system. It acts as a conduit for air, filters foreign particles, humidifies the inhaled air, provides structural support to keep the airway open and contributes to vocalization. Its proper functioning is crucial for maintaining efficient respiration and overall health.
Tracheal defects may occur as congenital anomalies or develop as acquired conditions following malignancy, trauma or infection. Small tracheal defects can be repaired using various open and endoscopic airway surgical techniques. However, long-segment tracheal defects, those involving one-third or more of the trachea, are associated with high morbidity and mortality, and a standard of care has not yet been established.
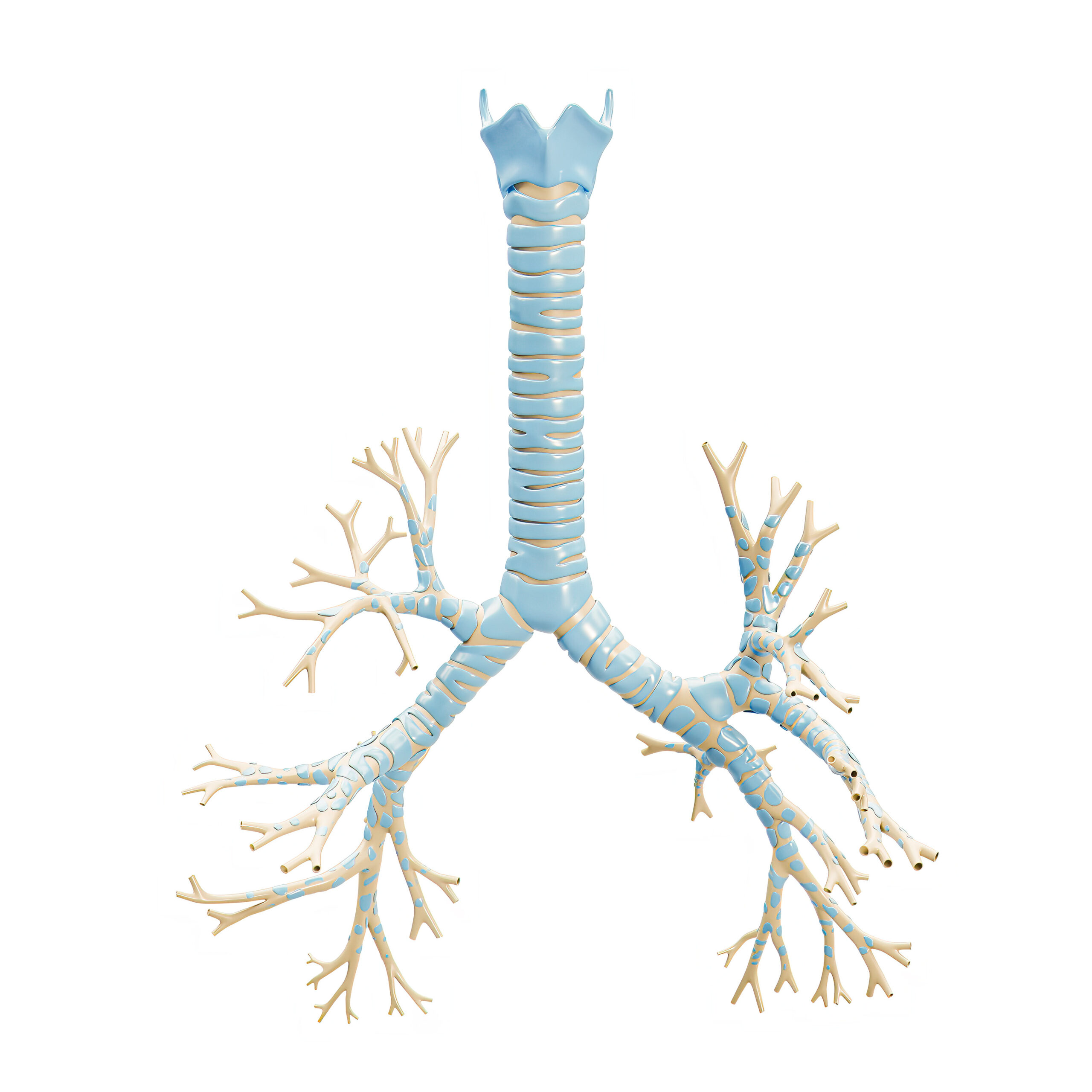
Unfortunately, no autologous tissues can be used for tracheal replacement. Furthermore, techniques using foreign materials for tracheal replacement and allograft tracheal transplantation have had limited success due to complications, including chronic infection, narrowing, rejection and malacia, an abnormal softening of tissue. However, emerging techniques in regenerative medicine and tissue engineering hold promise for repairing long-segment tracheal defects.
The life-threatening nature of long-segment tracheal defects has led to the compassionate use of tissue engineered tracheal grafts in the clinic, with the first report published in 2005. Since that time, the clinical application of tissue-engineered tracheal grafts has yielded some successes, but the optimal techniques for the generation of tissue-engineered tracheal grafts, in terms of safety and efficacy, have yet to be determined. Researchers at Nationwide Children’s Hospital are at the forefront of regenerative medicine, leading pre-clinical studies that are moving a new generation of tissue-engineered trachea grafts closer to clinical use.
Regenerative medicine is a relatively new, interdisciplinary science that aims to create tissues that are made from the patient’s own cells,” says Christopher Breuer, MD, director of the Center for Regenerative Medicine at the Abigail Wexner Research Institute at Nationwide Children’s, whose lab is designing a tissue-engineered vascular graft that will grow with pediatric patients. “In any field of reconstructive surgery, especially tracheal surgery, one of the main issues is when there is not enough of the patient’s own tissue for reconstruction. Tissue engineering does have real promise and significant potential for advancement here.”
Fundamentals of Tissue-engineered Trachea
Within the body, our cells make and secrete their own scaffolds, the extracellular matrix, and receive signals that stimulate normal growth and repair, explains Tendy Chiang, MD, FACS, principal investigator in the Center for Regenerative Medicine at Nationwide Children’s. Tissue engineering relies on those three primary components — a scaffold, cells and growth, and mechanical signaling factors that stimulate new tissue formation. The new functional tissue can then be used to restore, maintain or improve damaged tissues or organs.
“An ideal tracheal replacement will permit the replacement of diseased or absent tissue with a living construct capable of renewal and regeneration. To develop these grafts, we need mechanistic studies and basic science work,” says Dr. Chiang.
Dr. Chiang and his team focus their research on understanding how airway tissue repairs and regenerates itself. And they are using that information to design tissue-engineered tracheal grafts. They and their collaborators are taking a back-to-the-bench approach to define the mechanisms of cartilage formation, epithelialization and vascular regeneration. This method entails systematically analyzing how the tissue forms at a molecular and cellular level.
“These experiments allow us to rationally design strategies for making tracheal tissues,” says Dr. Breuer. “There is hope that we would then have the ability to help many more patients and positively change their lives.” The researchers have made steady progress. They recently developed techniques to create a tracheal graft that appears to overcome many of the complications associated with other graft types.
Partially Decellularized Tracheal Grafts
Decellularized tracheal scaffolds offer a potential solution for the repair of long-segment tracheal defects. However, complete decellularization of trachea is complicated by tracheal collapse. Recently, Dr. Chiang’s lab developed a method of creating tracheal grafts through partial decellularization. Partial decellularization is a way of removing the immunogenic cell types, which can cause the graft to be rejected, while preserving the chondrocytes and key proteins of the extracellular matrix, such as collagen, glycosaminoglycans, laminin and fibronectin. Retaining these cells and proteins allows the partially decellularized tracheal graft to maintain the mechanical properties of the trachea and also creates a natural scaffold for the regeneration of the epithelium.
In any field of reconstructive surgery, especially tracheal surgery, one of the main issues is when there is not enough of the patient’s own tissue for reconstruction.”
—Christopher Breuer, MD, director of the Center for Regenerative Medicine at Nationwide Children’s

“Decellularization is a method to strip all cells from donor tissue. Partial decellularization is very similar to conventional decellularization techniques, but it aims to preserve the cartilage and the cells within the cartilage while removing all of the cells around the cartilage,” explains Dr. Chiang.
In a 2021 Journal of Tissue Engineering publication, the Nationwide Children’s team along with collaborators at The Ohio State University created and characterized a partially decellularized tracheal scaffold in a mouse model of tracheal transplantation. They found that following partial decellularization, the graft maintained its integrity, including chondrocytes and predominant extracellular matrix proteins. After transplantation, they assessed the performance of the graft in vivo. The grafts formed a functional neo-epithelium and demonstrated restoration of native tracheal rigidity by the end of the study.
“We demonstrated that, unlike our previous experience with synthetic scaffolds, these grafts are able to support the growth of an epithelium that has all the cell types that are characteristic of the native trachea,” explained Dr. Chiang. “We believe this partially decellularized scaffold offers solution for long-segment tracheal defects, and it is an approach that is being adopted by more research groups. We are currently in the midst of additional preclinical testing of this graft.”
With further study of the partially decellularized tracheal grafts, the team confirmed that they are able to overcome another key challenge in the field: rejection by the graft recipient’s immune system. Earlier this year, they published a study in the journal Bioengineering & Translational Medicine demonstrating that partial decellularization eliminated allograft immunogenicity, with no histologic signs of rejection observed in mouse models following implantation of the graft. This represents a critical step toward clinical translation.
The researchers have extended their findings in a new article published in the journal NPJ Regenerative Medicine. They used a combination of cellular and genetic approaches to compare the neotissue that developed on the partially decellularized tracheal grafts in transplanted individuals with tissue in the native airway of individuals that did not undergo surgery and surgical controls. The study showed that the neotissue of the partially decellularized tracheal grafts was composed of the same cell populations found in the native trachea. Additionally, the regenerated epithelium and microvasculature, which included tubular blood vessels filled with red blood cells, persisted for at least 6 months.
The team also found that the graft attracted tissue-specific stem cells that maintain the tracheobronchial airway epithelium, called basal cells, and these stem cells exhibited normal proliferation and differentiation. The basal cells were able to differentiate into multiciliated and club cells that restored the mucociliated airway epithelium, implying mucosecretory clearance. As of the end of the study, none of the study animals exhibited signs of respiratory distress.
“There is a vast array of molecular tools that can be used in mouse models. These allow us to turn genes on and off to examine the roles of different proteins and to label cells to see where they are migrating in the tracheal grafts. It is a powerful system that allows us to study how all the components fit together,” adds Dr. Breuer.

We demonstrated that, unlike our previous experience with synthetic scaffolds, these grafts are able to support the growth of an epithelium that has all the cell types that are characteristic of native trachea.”
—Tendy Chiang, MD, FACS, principal investigator in the Center for Regenerative Medicine at Nationwide Children’s
Together, these findings demonstrate that partially decellularized tracheal grafts are not rejected and support neotissue formation within the recipient. The regenerated tracheal tissue recapitulates the structure and function of the host trachea, including spatially appropriate recellularization. The studies provide strong support for further evaluation of partially decellularized tracheal grafts in the next stages of preclinical study. “We have found that, in our mouse model, this graft is able to support regeneration of host-derived tissue, all the different components of a trachea, including regrowth of blood supply and regrowth of an epithelium that appears to be identical to the native trachea. That gives us hope of being able to create a graft that is able to respond to injury and grows with the patient,” explains Dr. Chiang.
Future Directions
Now that Dr. Chiang and his team have demonstrated that growth and restoration of the tracheal epithelium and microvasculature occur in the mouse model, the next step is to assess the performance of a partially decellularized tracheal graft in a large animal model.
The researchers also are interested in exploring the potential application of partially decellularized tracheal grafts in populations that have airway disease and in those who have had previous airway surgery. To do so, Dr. Chiang is collaborating with Susan Reynolds, PhD, a principal investigator in the Center for Perinatal Research at Nationwide Children’s, whose research is focused on stem cells that maintain the airway epithelium. Dr. Reynolds contributed to the previously mentioned studies of stem cell viability, migration and differentiation in the mouse models.
“We would like to understand how the presence of airway disease or previous airway surgery affects the stem cells of the host and their ability to regenerate tissue,” says Dr. Reynolds. “Some of our basic science work indicates that lung disease actually causes biological aging of the airway stem cell population, meaning the biological age of the cells is older than the chronological age. For instance, a 3-year-old patient could have a stem cell population that is similar to that of a 10-year-old patient, and vice versa. This can have implications for the ability of those stem cells to proliferate and differentiate.”
Dr. Reynolds and her team are using molecular techniques to estimate the biological age of patients’ cells, for example, assessing the absolute number of stem cells within the epithelium and determining the telomere length, which typically decreases as cells age. So far, her team has identified an inverse correlation between the biological age and the function of the stem cell population (the ability to proliferate and to produce differentiated cell types). They hope that information will help identify patients who would be more or less likely to do well if they were to receive a partially decellularized tracheal graft.
Some of our basic science work indicates that lung disease actually causes biological aging of the airway stem cell population, meaning the biological age of the cells is older than the chronological age. For instance, a 3-year-old patient could have a stem cell population that is similar to that of a 10-year-old patient, and vice versa.”
—Susan Reynolds, PhD, principal investigator in the Center for Perinatal Research at Nationwide Children’s

Collaboration: The Key to Future Success
The future of tissue-engineered tracheal grafts hinges on the collaborative and translational commitment of the researchers involved. In the Center for Regenerative Medicine at Nationwide Children’s, teams are advancing the field of regenerative medicine and collaborating with a global network of biologists, clinicians, engineers and specialists in other related fields.
“The progress we have made thus far has been dependent on the multidisciplinary team of people working on the project and the great support from Nationwide Children’s,” shares Dr. Reynolds. “These are two levels of support that we have at Nationwide Children’s that do not exist at all institutions, making the hospital a unique place to do this work.”
“It is just a wonderful research ecosystem that we have here at Nationwide Children’s. The kinds of studies being done are enabled by the facilities that the institution is supplying, such as a surgical suite and imaging facilities for animal research that are on par with what would be used for studies in humans,” adds Dr. Chiang. “There are a lot of opportunities for truly multidisciplinary collaboration. We have clinicians working alongside scientists, as well as veterinary staff and engineers. Our success is really driven by the presence of these resources and the spirit of collaboration.”
This story also appeared in the Fall 2023 print issue. Download the full issue.
References
- Chiang T, Pepper V, Best C, Onwuka E, Breuer CK. Clinical Translation of Tissue Engineered Trachea Grafts. Annals of Otology, Rhinology & Laryngology. 2016 Nov; 125(11):873-885.
- Liu L, Dharmadhikari S, Shontz KM, Tan ZH, Spector BM, Stephens B, Bergman M, Manning A, Zhao K, Reynolds SD, Breuer CK, Chiang T. Regeneration of Partially Decellularized Tracheal Scaffolds in a Mouse Model of Orthotopic Tracheal Replacement. Journal of Tissue Engineering. 2021 Jun 6; 12:20417314211017417.
- Tan ZH, Liu L, Dharmadhikari S, Shontz KM, Kreber L, Sperber S, Yu J, Byun WY, Nyirjesy SC, Manning A, Reynolds SD, Chiang T. Partial Decellularization Eliminates Immunogenicity in Tracheal Allografts. Bioengineering & Translational Medicine. 2023; 8(5):e10525.
- Tan ZH, Dharmadhikari S, Liu L, Yu J, Shontz KM, Stack JT, Breuer CK, Reynolds SD, Chiang T. Regeneration of Tracheal Neotissue in Partially Decellularized Scaffolds. NPJ Regenerative Medicine. 2023 Jul 12; 8(1):35.
Image credits: Adobe Stock (illustration); Nationwide Children’s (portraits)
About the author
Lauren Dembeck, PhD, is a freelance science and medical writer based in New York City. She completed her BS in biology and BA in foreign languages at West Virginia University. Dr. Dembeck studied the genetic basis of natural variation in complex traits for her doctorate in genetics at North Carolina State University. She then conducted postdoctoral research on the formation and regulation of neuronal circuits at the Okinawa Institute of Science and Technology in Japan.
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/January 29, 2019
- Posted In:
- Clinical Updates
- Features
- Research