Findings Show TEVG Stenosis Spontaneously Resolves
Findings Show TEVG Stenosis Spontaneously Resolves https://pediatricsnationwide.org/wp-content/uploads/2020/10/TEVG-Stenosis-.jpg 600 400 Katie Brind'Amour, PhD, MS, CHES Katie Brind'Amour, PhD, MS, CHES https://pediatricsnationwide.org/wp-content/uploads/2021/03/Katie-B-portrait.gif- October 01, 2020
- Katie Brind'Amour, PhD, MS, CHES
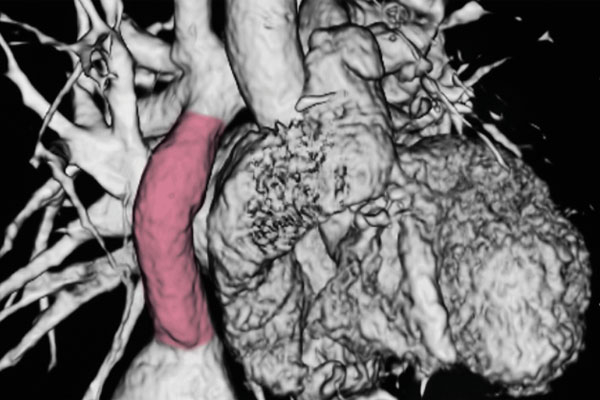
The complication that halted a clinical trial for tissue-engineered vascular grafts for children with congenital heart disease may reverse spontaneously without clinical complications.
Based on promising laboratory and animal modeling of a biodegradable scaffold seeded with a patient’s own cells, a clinician-scientist research team now based at Nationwide Children’s Hospital initiated a pediatric tissue engineered vascular graft (TEVG) trial in Japan for children requiring the Fontan procedure for univentricular hearts. After its high success rate — with only 1 in 25 patients developing serious stenosis, which was successfully treated — the group launched a similar study in the United States.
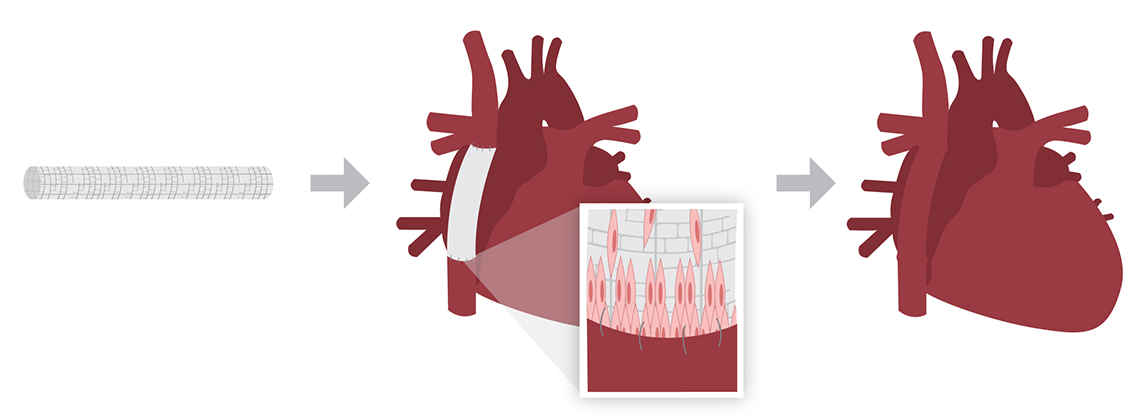
Tissue engineered vascular grafts use cells seeded on a three-dimensional biodegradable scaffold. The seeded scaffold is placed as a conduit during the Fontan surgery. Over time, the scaffold disappears, leaving a natural vessel composed of the child’s own cells.
Unfortunately, when 3 of the first 4 patients developed postoperative stenosis requiring balloon angioplasty, the study was terminated. All patients were safely treated and remain well several years after the trial.
To find out why the U.S. trial’s results differed so drastically from Japan’s, the investigators initiated robust computer modeling and found a surprising suggested explanation: early stenosis — a result of the body’s inflammatory response and the scaffold’s mechanical properties — may reverse on its own. Tests on sheep confirmed the computer model’s findings. Stenosis reversed spontaneously in time, without clinical complications.
According to the research, when the TEVG is implanted, stenosis develops due to inflammation, as the body recruits cells and builds new tissue on the scaffold. When the immune reaction calms, stenosis resolves, and the graft is replaced with a new, natural blood vessel that is virtually indistinguishable from native tissue.
“It’s possible that this exact phenomenon occurred in the Japanese trial, but was largely missed due to post-surgical imaging timing and different criteria for angioplasty,” says Christopher Breuer, MD, director of the Center for Regenerative Medicine and Endowed Chair in Surgical Research at Nationwide Children’s and director of Tissue Engineering at The Ohio State University Wexner Medical Center.
Dr. Breuer and Toshiharu Shinoka, MD, PhD, co-director of the Tissue Engineering Program at Nationwide Children’s jointly lead the team that designed the TEVG used, ran the trials and published the latest results from the computer and animal modeling. When the team reexamined available imaging from the Japanese trial, they found a few cases where narrowing was observed but monitored via imaging only. Most resolved without intervention.
The team is launching a new TEVG trial at Nationwide Children’s in 2020, with different stenosis monitoring and intervention criteria designed to accommodate the likely scenario of temporary, asymptomatic narrowing with spontaneous resolution. It is the team’s hope that TEVG will successfully, permanently treat single-ventricle congenital heart disease.
This story appeared in the Fall/Winter 2020 print issue. Download the issue now.
References
- Drews JD, Pepper VK, Best CA, Szafron JM, Cheatham JP, Yates AR, Hor KN, Zbinden JC, Chang YC, Mirhaidari GJM, Ramachandra AB, Miyamoto S, Blum KM, Onwuka EA, Zakko J, Kelly J, Cheatham SL, King N, Reinhardt JW, Sugiura T, Miyachi H, Matsuzaki Y, Breuer J, Heuer ED, West TA, Shoji T, Berman D, Boe BA, Asnes J Galantowicz M, Matsumura G, Hibino N, Marsden AL, Pober JS, Humphrey JD, Shinoka T, Breuer CK. Spontaneous reversal of stenosis in tissue-engineered vascular grafts. Science Translational Medicine. 2020 Apr 1;12(537):eaax6919.
- Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T. Late-term results of tissue-engineered vascular grafts in humans. Journal of Thoracic and Cardiovascular Surgery. 2010;139:431–436.e2.
Image credits: Nationwide Children’s
About the author
Katherine (Katie) Brind’Amour is a freelance medical and health science writer based in Pennsylvania. She has written about nearly every therapeutic area for patients, doctors and the general public. Dr. Brind’Amour specializes in health literacy and patient education. She completed her BS and MS degrees in Biology at Arizona State University and her PhD in Health Services Management and Policy at The Ohio State University. She is a Certified Health Education Specialist and is interested in health promotion via health programs and the communication of medical information.
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 27, 2014
-
Katie Brind'Amour, PhD, MS, CHEShttps://pediatricsnationwide.org/author/katie-brindamour-phd-ms-ches/April 28, 2014
- Posted In:
- In Brief





