From Clinician Ideas to Commercially-Available Clinical Devices
From Clinician Ideas to Commercially-Available Clinical Devices https://pediatricsnationwide.org/wp-content/themes/corpus/images/empty/thumbnail.jpg 150 150 Kevin Mayhood Kevin Mayhood https://secure.gravatar.com/avatar/bd57a8b155725b653da0c499ae1bf402?s=96&d=mm&r=g- January 18, 2020
- Kevin Mayhood
Pressure wounds were a common complication following a tracheostomy, often resulting in advanced-stage wounds, national studies showed. Nationwide Children’s Hospital was no different but Kris Jatana, MD, and Charles Elmaraghy, MD, surgeons in the Department of Otolaryngology, knew they could improve these outcomes.
Brendan Boyle, MD, and Alex Green, DO, were fellows in the Division of Gastroenterology, Hepatology and Nutrition, who were frequently called by the emergency department to remove food boluses stuck in a child’s esophagus, most commonly related to swelling of the esophageal lining. The available devices rarely grasped the bolus whole, instead breaking off pieces and making removal a long process. They had ideas for a better tool.
We thought pressure wounds could be prevented with consistent barrier protection between the plastic tracheostomy tube and the skin.
— Dr. Jatana

The teams turned to the Office of Technology Commercialization (OTC) and discussed what they had in mind. At the office’s encouragement, they applied for grants and each team received $25,000 from the office’s Technology Development Fund in 2011.
With the OTC’s help, their intellectual properties are patented, their technologies licensed and the devices they’d envisioned are now being used in hospitals across the country.
Bringing the Comfort Collar to Market
“We thought pressure wounds could be prevented with consistent barrier protection between the plastic tracheostomy tube and the skin,” says Dr. Jatana, now director of Pediatric Otolaryngology Quality Improvement. “What was historically done — placing gauze under the tube as a barrier — is problematic. The gauze not only obstructs your view of the site, but gets wet, irritating the skin, which is the start of the wound.”
Drs. Jatana and Elmaraghy considered materials used to prevent friction injuries and looked at those used in sports. They believed neoprene would be a soft but sturdy water-resistant barrier and bought a neoprene wetsuit from a sporting goods store. They created a prototype collar from the material in Dr. Elmaraghy’s living room and began testing.
“We spent several years going through prototypes and tweaking the design to get a universal fit for all tracheostomy tubes and designed a more secure Velcro attachment to prevent the tube from accidentally being dislodged,” Dr. Jatana says. “We worked with several potential manufacturers and found Marpac, based in Albuquerque, a good partner.”
The tracheostomy collar is the result of a great partnership between the OTC and clinical medicine, providing surgeons opportunities to apply practical knowledge and translating it to a product changing outcomes for patients.
— Dr. Elmaraghy

The company makes tracheostomy collars and had the machinery and most of the materials required, making the device a good fit for the business, says Dave Mayberry, Marpac’s product development manager. With the assistance of the OTC, Marpac licensed the technology and now manufactures and sells it as the Comfort Collar to hospitals in a dozen states, the U.K., Canada and the United Arab Emirates, Mayberry says. The Comfort Collar is the first clinical device developed by a clinician at Nationwide Children’s to be commercially available.
Dr. Elmaraghy, who is now chief of the Department of Otolaryngology, says “The tracheostomy collar is the result of a great partnership between the OTC and clinical medicine, providing surgeons opportunities to apply practical knowledge and translating it to a product changing outcomes for patients.”
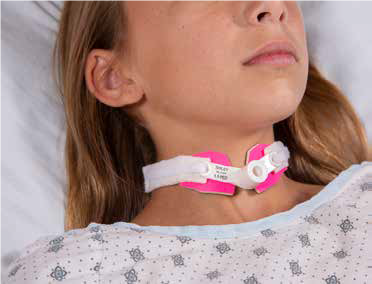
Photo credit: Marpac
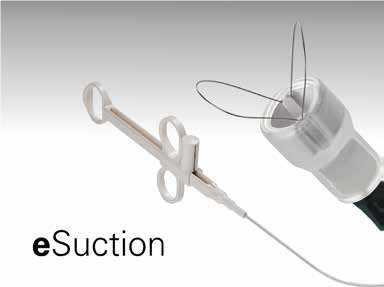
Photo Credit: Endo-Therapeutics
Designing the eSuction Solution
With their technology development funding, Dr. Boyle, now an associate professor of Clinical Pediatrics and quality improvement director in the Division of Gastroenterology, Hepatology and Nutrition, and Dr. Green, now a gastroenterologist at Lurie Children’s Hospital of Chicago, reached out to engineering firms to help them develop a prototype.
After the prototype had been tested in a large animal model, Kyle Murrah, PhD, senior licensing associate in the OTC, negotiated the license with Endo-Therapeutics, in Clearwater, Florida.
“Endo-Therapeutics saw this was a technology solving an unmet need,” Murrah says.
The final product, called eSuction, is placed on the tip of an endoscope. Instead of working like forceps, the device has two small wire loops that the operator slides past either side of the bolus. A gentle pull on the loops wraps the bolus and a vacuum seals it to the tip for removal.
Gastroenterology faculty and staff at Nationwide Children’s were recently trained to use eSuction. “We think it will help shorten the duration of the cases which should mean shortened anesthesia times and reduced trauma to the esophagus,” Dr. Boyle says.
A Future Full of New Devices and Technologies
For Drs. Jatana and Elmaraghy, the Comfort Collar experience was just the start. They are now working with the OTC on other devices they believe will improve patient safety during surgical procedures.
And while Drs. Jatana, Elmaraghy, Boyle and Green were the first clinicians to bring devices to the market from Nationwide Children’s, many others are following suit.
Dr. Boyle recommends others with ideas for devices pursue them. “I don’t think that we, as the frontline workers, realize how meaningful our insight can be… We can often best recognize the deficiencies in the currently available tools and provide needed insight about the areas for improvement.”
“Before this experience, I knew nothing about making a medical device,” Dr. Boyle says. “But the OTC staff guided us through the entire development process – and with our efforts combined and the right partners in place, our idea became a successful product that is now able to help kids in our institution and others.”
About the author
-
Kevin Mayhoodhttps://pediatricsnationwide.org/author/kevin-mayhood/
-
Kevin Mayhoodhttps://pediatricsnationwide.org/author/kevin-mayhood/April 25, 2015
-
Kevin Mayhoodhttps://pediatricsnationwide.org/author/kevin-mayhood/April 25, 2015
-
Kevin Mayhoodhttps://pediatricsnationwide.org/author/kevin-mayhood/April 25, 2015
- Posted In:
- Clinical Updates
- Research