Tissue Engineering and Fetal Medicine: A New Frontier for Congenital Heart Disease
Tissue Engineering and Fetal Medicine: A New Frontier for Congenital Heart Disease https://pediatricsnationwide.org/wp-content/uploads/2019/04/Insight-Left-Side_FINAL-for-web-header-1024x575.jpg 1024 575 Abbie Miller Abbie Miller https://pediatricsnationwide.org/wp-content/uploads/2023/05/051023BT016-Abbie-Crop.jpg- May 10, 2023
- Abbie Miller
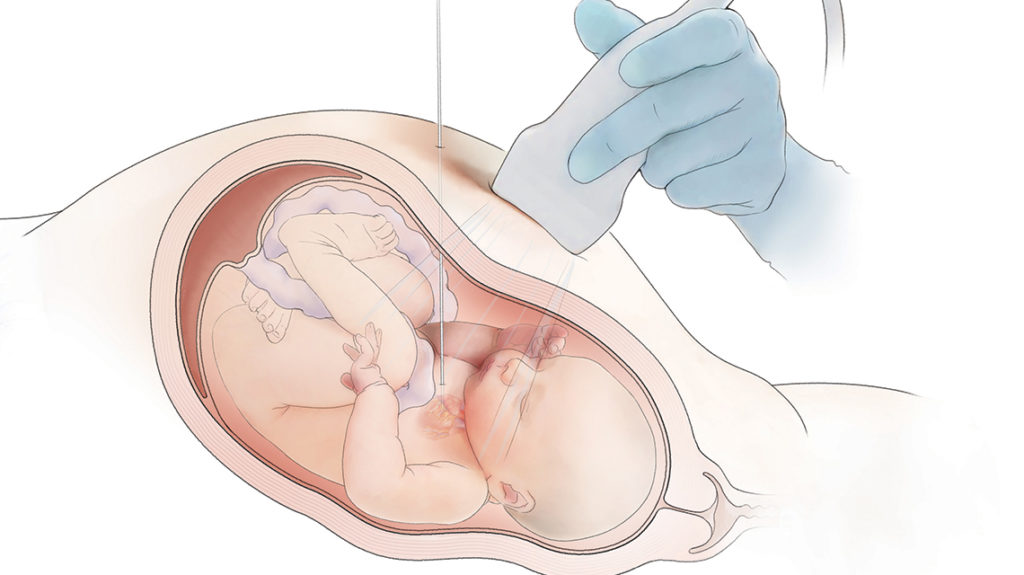
Procedures that utilize cardiac catheterization to improve fetal heart development are often successful, but they are not without risk. And even if they can successfully prevent the development of single ventricle disease, there is always ongoing heart valve disease (HVD). HVD may not be as life-threatening as single ventricle disease, but it requires lifelong care.
Fetal Approach to Preventing Single Ventricle Disease
Hypoplastic left heart syndrome (HLHS) is a type of single ventricle congenital heart disease in which a baby is born with an underdeveloped left ventricle. Other parts of the heart, such as the aorta and mitral valve, are also affected. Children with HLHS face many surgeries and heart catheterizations to reroute flow to the body. Patients with HLHS will eventually face heart failure and need a heart transplant.
HLHS can be genetic, but the cause is still largely unknown. At least some cases originate from a narrowed aortic valve that changes the forces exerted by the blood on the developing tissues of the left ventricle, leading to underdevelopment.
In fetal balloon aortic valvuloplasty, a catheter is inserted through the mother’s abdomen, across the fetal chest wall and into the fetus’s heart. A balloon is inflated to expand the narrowed valve and improve blood flow. Research has shown that this can help the left ventricle form more normally.
“The goal of the minimally invasive fetal procedure is to attempt to reverse the developing HLHS and decrease the number of open-heart surgeries and morbidity for the child later in life,” says Aimee Armstrong, MD, director of Cardiac Catheterization and Interventional Therapies at Nationwide Children’s Hospital. “In some cases, the procedure completely reverses evolving HLHS, to the point that an open-heart surgery may never be needed, which is the best possible outcome.”
Addressing Heart Valve Disease
Even if the balloon procedure prevents HLHS, the aortic valve will still be abnormal. Children with poorly functioning heart valves also need surgeries and heart catheterizations over the course of their lives. But the outcomes for HVD are generally better than those for HLHS.
But what if, instead of just ballooning the valve, doctors could deliver a replacement valve via a catheter, preventing HLHS and delivering a normal valve? And, what if that replacement heart valve had growth potential – serving as a long-term solution instead of needing to be replaced as the child grows?
That’s the vision motivating a team of researchers from Nationwide Children’s Hospital and around the world.
A study published in the Journal of American College of Cariology: Basic to Translational Science describes preclinical work to develop a tissue engineered heart valve that could be delivered by catheter to a fetal heart.
Led by researchers from the Center for Regenerative Medicine and The Heart Center at Nationwide Children’s Hospital and University Hospitals and Rainbow Babies and Children’s Hospital in Cleveland, the success of the preclinical work is supported by the collaboration of experts in biomechanics, bioengineering, regenerative medicine, fetal development, maternal-fetal medicine, and interventional catheterization.
Tissue Engineered Heart Valves
Christopher Breuer, MD, and Toshiharu Shinoka, MD, PhD, created the first tissue engineered heart valve more than 20 years ago at Harvard University. Their work pivoted to focus on moving tissue engineered vascular grafts (TEVGs) into the clinic. Their TEVGs have been in clinical trials since 2012 and they have been implanted in 34 children to date.
Now, Dr. Breuer is shifting his focus back to heart valves, inspired in part by the opportunity for collaboration and innovation with the Fetal Center at Nationwide Children’s.
“When you consider the environment of the womb, where a fetus is growing and developing and the conditions are ideal for that, the possibilities of tissue engineering are quite exciting,” says Dr. Breuer. “The womb is the ideal bioreactor. It’s the ultimate healing environment.”
At 23 weeks of gestation, which is when fetal aortic valvuloplasty is usually performed, the heart is about three fourths of an inch long or the size of a small strawberry. The aortic valve is even smaller, being just a few millimeters in diameter.
Designing a valve that can fit inside a stent to be delivered in such a small space is — pun intended — no small feat.
“The work around tissue engineered heart valves for the fetal heart has been a back and forth between the biological advances and the technical developments,” says Dr. Breuer. “Many of our hurdles have been on the technical, bioengineering side of the work. We’re working on a variety of prototypes in preclinical studies and collaborations to find the best solution.”
In their JACC study, Dr. Breuer and team’s approach used a fully biodegradable fetal valve made from electrospun polycaprolactone leaflets, which they attached to a biodegradable zinc-aluminum alloy stent. The valve was tested in vitro and in vivo and showed feasibility in a large animal model.
“While we are still in preclinical studies, we are very excited about the trajectory and opportunity present in this work,” says Dr. Breuer. “Working with our international collaborators, we are continuing to refine our design and engineering, while building our case for potential clinical studies.”
Reference:
Zakko J, Blum KM, Drews JD, Wu Y-L, Hatoum H, Russell M, Gooden S, Heikemper M, Conroy O, Kelly J, Carey S, Sacks M, Texter K, Ragsdale E, Strainic J, Bocks M, Wang Y, Dasi LP, Armstrong AK, Breuer C. Development of tissue engineered heart valves for percutaneous transcatheter delivery in a fetal ovine model. JACC: Basic to Translational Science. 2020;5(8).
Image credit: Nationwide Children’s
About the author
Abbie (Roth) Miller, MWC, is a passionate communicator of science. As the manager, medical and science content, at Nationwide Children’s Hospital, she shares stories about innovative research and discovery with audiences ranging from parents to preeminent researchers and leaders. Before coming to Nationwide Children’s, Abbie used her communication skills to engage audiences with a wide variety of science topics. She is a Medical Writer Certified®, credentialed by the American Medical Writers Association.
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/






