Novel Nitric Oxide-Mediated Mechanism Facilitates Calcific Aortic Valve Disease
Novel Nitric Oxide-Mediated Mechanism Facilitates Calcific Aortic Valve Disease https://pediatricsnationwide.org/wp-content/uploads/2021/03/4dc2b000-8f21-11e7-aa62-e89a8fb23f02-1024x752.jpg 1024 752 Lauren Dembeck Lauren Dembeck https://pediatricsnationwide.org/wp-content/uploads/2021/03/Dembeck_headshot.gif- March 02, 2021
- Lauren Dembeck
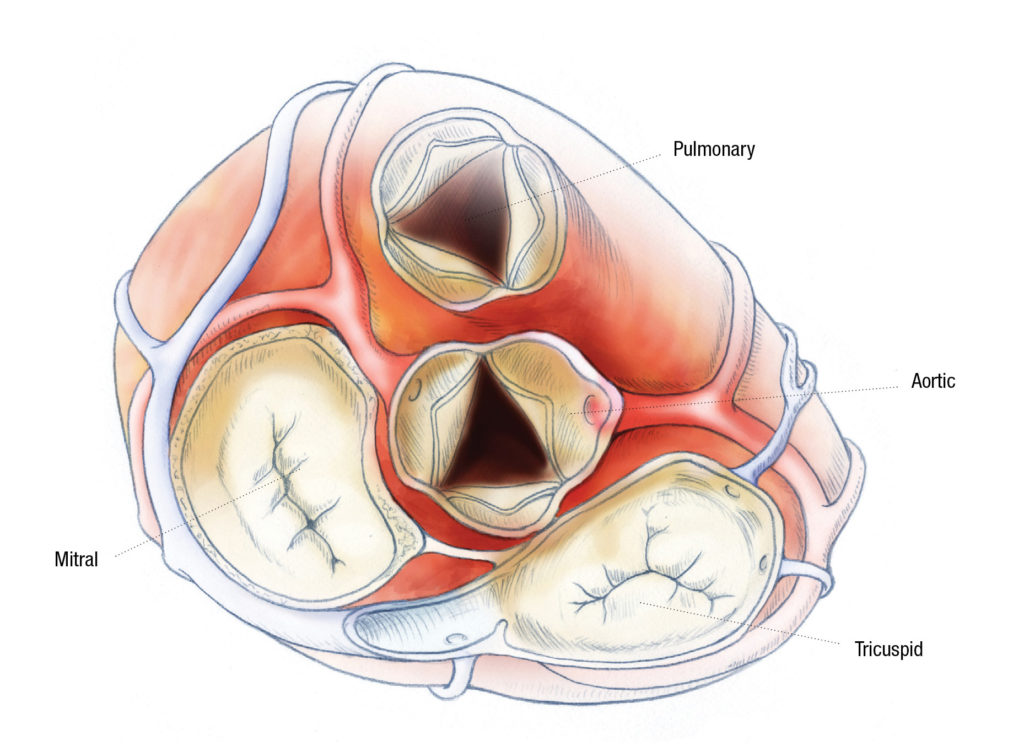
The novel pathway could be targeted to prevent or reverse aortic valve calcification in humans.
Researchers have recently described a novel nitric oxide-mediated mechanism in calcific aortic valve disease that involves the ubiqutin-proteasome pathway, and its modulation in animal models was shown to cause aortic valve calcification.
The pathway could potentially be modulated to prevent or treat calcific aortic valve disease, an increasingly prevalent disease among the growing aging population that individuals born with a bicuspid aortic valve are predisposed to developing as adults.
The findings were published in Science Advances by a multicenter team led by Vidu Garg, MD, director of the Center for Cardiovascular Research and the Nationwide Foundation Endowed Chair in Cardiovascular Research at Nationwide Children’s Hospital.
“The proposed mechanisms are initiated by injury of the endothelial cells lining the aortic valve leaflets that ultimately drives a gene expression program within the valve cells leading to calcification,” says Dr. Garg.
In a series of experiments, the team showed that the novel mechanism works via a post-translational S-nitrosylation of the USP9X protein, which deubiquitinates and stabilizes a NOTCH1 ligand found in endothelial cells of the heart valve called MIB1. When MIB1 is stable, it can activate NOTCH1 signaling in neighboring cells, inhibiting calcification. When MIB1 is unstable, RUNX2, a marker of osteoblast differentiation, is expressed and calcification ensues.
The team first confirmed that nitric oxide and an S-nitrosylating agent can both rescue spontaneous calcification in aortic valve cells. Next, single cell RNA-sequencing revealed that nitric oxide and the S-nitrosylating agent regulate the NOTCH1 pathway, while no components of the cyclic GMP pathway showed changes with either treatment.
“Current evidence in the field demonstrates that nitric oxide prevents calcification through the cyclic GMP pathway. Our data demonstrates that the activation of NOTCH1 signaling pathway is functioning through this post-translational mechanism,” explains Dr. Garg.
The team used mass spectrometry to identify which proteins were S-nitrosylated in aortic valve cells after nitric oxide exposure. Ubiquitin-specific processing was the most enriched pathway, and one ubiquitin-specific processing protease, USP9X, caught their interest because it had previously been connected to NOTCH signaling.
This led the researchers to develop a mouse model to determine if USP9X deficiency leads to calcific aortic valve disease in vivo. Indeed, when the mice lack USP9X in their endothelial cells, they show evidence of functional aortic valve stenosis and regurgitation along with thickened leaflets, indicative of aortic valve disease.
“Previously in this field, we did not have a highly penetrant and easily reproducible mouse model to study the role of NOTCH1 signaling in calcific aortic valve disease,” says Uddalak Majumdar, PhD, a post-doctoral scientist at Nationwide Children’s and lead author of the study. “Now, we can study calcification in vivo, opening up the opportunity for preclinical testing of drug targets.”
Lastly, the team examined gene and protein expression levels in explanted aortic valve tissues from patients who had surgical valve replacement due to aortic stenosis. In all calcified aortic valve leaflets, the amount of S-nitrosylated USP9X was reduced, and its level was inversely correlated with increasing amounts of calcification.
The findings have implications for drug development. “This is important because current drugs that only enhance the cyclic GMP pathway may not be as effective in calcific aortic valve diseases,” explains Dr. Garg.
“This is the first time the ubiquitin-proteasome pathway has been linked to calcific aortic valve disease. Importantly, this pathway is targetable; studies of cancer patients are already testing drug targets in this pathway, which with further investigation may ultimately be applicable in calcific aortic valve disease,” says Dr. Majumdar.
The team plans to begin testing some of these potential medical treatments in their newly developed mouse model for calcific aortic valve disease.
Reference:
Majumdar U, Manivannan S, Basu M, et al. Nitric oxide prevents aortic valve calcification by S-nitrosylation of USP9X to activate NOTCH signaling. Science Advances. 2021;7(6):eabe3706. Published 2021 Feb 5. doi:10.1126/sciadv.abe3706
Image credit: Nationwide Children’s
About the author
Lauren Dembeck, PhD, is a freelance science and medical writer based in New York City. She completed her BS in biology and BA in foreign languages at West Virginia University. Dr. Dembeck studied the genetic basis of natural variation in complex traits for her doctorate in genetics at North Carolina State University. She then conducted postdoctoral research on the formation and regulation of neuronal circuits at the Okinawa Institute of Science and Technology in Japan.
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/
-
Lauren Dembeckhttps://pediatricsnationwide.org/author/lauren-dembeck/January 29, 2019
- Posted In:
- In Brief