Magnetically Controlled Limb Lengthening Devices Safe for Patients With Programmable Implantable Devices, Study Shows
Magnetically Controlled Limb Lengthening Devices Safe for Patients With Programmable Implantable Devices, Study Shows https://pediatricsnationwide.org/wp-content/uploads/2022/08/011218ds0720-1024x683.jpg 1024 683 Erin Gregory Erin Gregory https://secure.gravatar.com/avatar/?s=96&d=mm&r=g- March 12, 2024
- Erin Gregory

Recent study evaluated the safety and efficacy of using magnetically controlled intramedullary nails in patients with programmable implantable devices, shedding light on a previously unexplored area of orthopedic surgery.
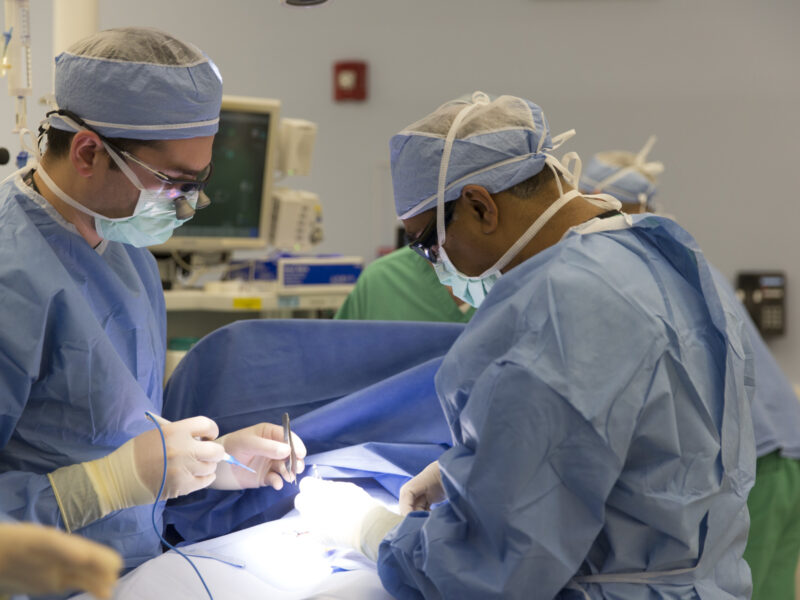
In a pivotal study published in the Strategies in Trauma and Limb Reconstruction, led by Christopher A. Iobst, MD, a pediatric orthopedic surgeon at Nationwide Children’s Hospital, researchers, in collaboration with other institutions, have unveiled a significant breakthrough in limb reconstruction surgery. The study investigates the safety and efficacy of utilizing magnetically controlled intramedullary nails in patients with programmable implantable devices, shedding light on a previously unexplored area of orthopedic surgery.
A Comprehensive Approach to Limb Lengthening Procedures
Traditionally, limb lengthening procedures have often relied on external fixators, which come with inherent discomfort and infection risks. However, the introduction of magnetically driven intramedullary limb lengthening devices has offered a more patient-friendly alternative. Despite its advantages, concerns lingered regarding potential interference with implanted programmable devices like cardiac pacemakers.
The study involved four patients with limb length discrepancies seeking correction. The patients each had programmable devices: one patient had a VP shunt, one had a gastric pacer, and two had cardiac pacemakers. Extensive counseling and consultations with medical teams preceded the decision to move forward with surgery. In turn, osteoplasty was performed, and magnetically driven intramedullary lengthening nails were inserted.
“All patients achieved their targeted limb lengths, with successful consolidation and subsequent nail removal,” Dr. Iobst noted. “No instances of malfunction in the implanted devices during the distraction phase were observed, highlighting the procedure’s safety and effectiveness.”
By following strict protocol to keep the devices away from one another, the team reported that they did not encounter any disruption of function of the implantable devices.
“When handling the magnet in the handheld device, it was always kept a maximum distance from the location of the implanted device. Magnet location in the lengthening nail was also considered and influenced surgical decision-making,” elaborates Dr. Iobst.
Meticulous planning and consideration were essential in selecting the appropriate patients for this approach.
“We started with less potential life-threatening implantable device issues to determine that this technique was feasible,” Dr. Iobst says. “Once we validated that this was safe, we moved on to riskier scenarios, gradually ramping up to more dangerous devices rather than just jumping in without prior experience or precautions.”
Making Strides in Orthopedic Surgery
The successful outcomes observed in this study not only demonstrate the safety and efficacy of magnetically controlled intramedullary nails but also pave the way for future advancements in limb reconstruction surgery.
“This research marks a significant stride forward in orthopedic surgery,” says Dr. Iobst. “By demonstrating the viability of this technique in patients with programmable implantable devices, we open doors to expanded treatment options and improved outcomes for individuals with limb length discrepancies.”
The study’s findings offer hope for patients with limb length discrepancies and programmable implantable devices. Through careful planning, adherence to safety protocols, and innovative approaches, orthopedic surgeons can now expand treatment options and improve outcomes in limb reconstruction surgery.
Reference:
Iobst CA, Hatfield DN, Forro SD, Quinnan SM. Magnetically Driven Intramedullary Limb Lengthening in Patients with Pre-existing Implanted Programmable Devices: A Case Series. Strategies Trauma Limb Reconstr. 2023;18(2):111-116. doi:10.5005/jp-journals-10080-1590
About the author
-
Erin Gregoryhttps://pediatricsnationwide.org/author/erin-gregory/September 27, 2023
-
Erin Gregoryhttps://pediatricsnationwide.org/author/erin-gregory/
-
Erin Gregoryhttps://pediatricsnationwide.org/author/erin-gregory/
-
Erin Gregoryhttps://pediatricsnationwide.org/author/erin-gregory/January 4, 2024
- Posted In:
- Clinical Updates
- In Brief
- Research