Targeting Bacterial Biofilm Linchpin Prevents and Treats Recalcitrant Biofilm-Mediated Infections
Targeting Bacterial Biofilm Linchpin Prevents and Treats Recalcitrant Biofilm-Mediated Infections https://pediatricsnationwide.org/wp-content/uploads/2020/04/biofilm-header-1024x575.jpg 1024 575 Abbie Miller Abbie Miller https://pediatricsnationwide.org/wp-content/uploads/2023/05/051023BT016-Abbie-Crop.jpg- July 07, 2020
- Abbie Miller
A new study highlights two approaches with substantive efficacy and potential for broad application to combat biofilm-mediated diseases.
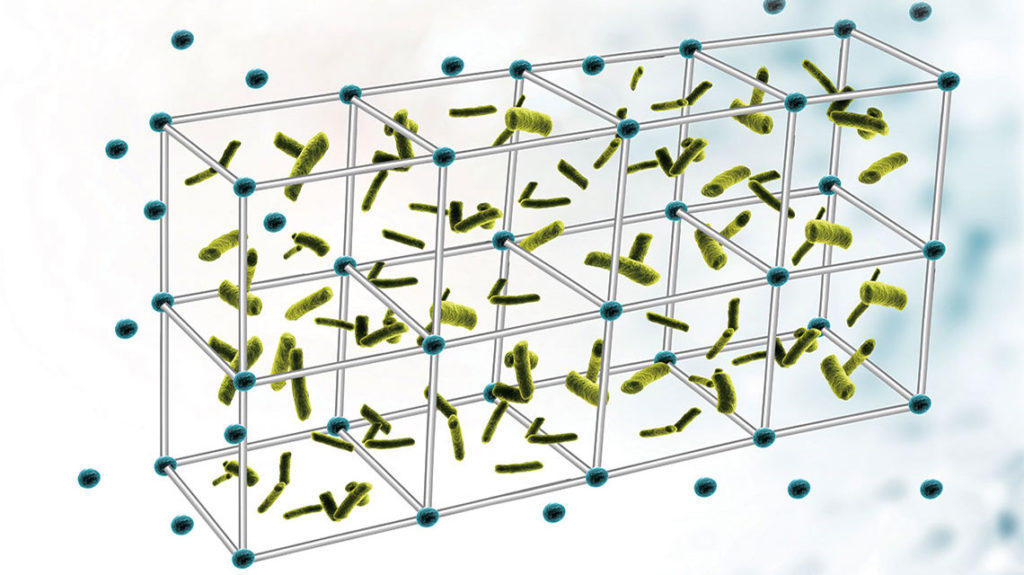
Chronic and recurrent bacterial diseases are treatment-resistant due to the ability of the pathogens to establish biofilms, which act as fortresses built of extracellular DNA and proteins to protect populations of the bacteria.
For more than 11 years, researchers Lauren Bakaletz, PhD, and Steve Goodman, PhD, have been working to understand and dismantle biofilms.
“Biofilms are involved in the majority of chronic and recurrent bacterial diseases in the respiratory tract, oral cavity, gastrointestinal tract and urogenital tract,” says Dr. Bakaletz, director of the Center for Microbial Pathogenesis and vice president of Basic Sciences at the Abigail Wexner Research Institute at Nationwide Children’s (AWRI). “Our work has been to understand the structure and function of biofilms, and ultimately, to find a novel approach to prevent or eradicate them.”
Earlier this year, Drs. Bakaletz, Goodman and their team reported in Proceedings of the National Academy of Sciences (PNAS) the role of the DNABII family of bacterial DNA-binding proteins as the “linchpin” of the biofilm structures.
In their latest paper, published in EBioMedicine, they demonstrate how using a new platform technology of DNABII-directed antibodies can rapidly disrupt existing biofilms– and prevent them from forming at all.
“This approach attacks a feature common to all biofilms – it’s worked on every type of biofilm we’ve tested,” says Dr. Goodman, study author and director of the Oral GI Microbiology Research Affinity Group in the Center for Microbial Pathogenesis at AWRI. “We believe this technology has the potential to resolve infections characterized by communities of heterogenous bacteria where other single-pathogen approaches have failed.”
In a preclinical model of otitis media caused by nontypeable Haemophilus inluenzae (NTHI), a monoclonal antibody against a chimeric peptide immunogen designed to target specific regions of the DNABIII proteins, as well as its antigen-binding fragment, was used to disrupt established biofilms. In the preclinical model, this approach resolved the NTHI-induced disease and eradicated the biofilms from the middle ears.
Next, the team used the chimeric peptide immunogen as a preventative immunization, which induced antibodies that prevented biofilm formation and disease development in a viral-bacterial coinfection model.
Finally, the team humanized a monoclonal antibody against the chimeric peptide immunogen, then characterized and validated that it maintained therapeutic efficacy both in vitro and in vivo.
“With this novel approach, we have an opportunity to shift the paradigm for clinical management of a multitude of diseases characterized by recalcitrant biofilms that evade effective intervention today,” says Dr. Bakaletz. “Our results from this study are highly supportive of entry into both preventative and therapeutic clinical trials.”
The technology described in the study has been licensed to Clarametyx Biosciences, Inc., a preclinical stage biotechnology company developing targeted, immune-enabling biologic therapies to counter persistent infections associated with biofilms. Drs. Bakaletz and Goodman are co-inventors of the technology and co-chairs of the Clarametyx scientific advisory board.
Reference:
Novotny LA, Goodman SD, Bakaletz LO. Targeting a bacterial DNABII protein with a chimeric peptide immunogen or humanized monoclonal antibody to prevent or treat recalcitrant biofilm-mediated infections. EBioMedicine.
Image credit: Nationwide Children’s
About the author
Abbie (Roth) Miller, MWC, is a passionate communicator of science. As the manager, medical and science content, at Nationwide Children’s Hospital, she shares stories about innovative research and discovery with audiences ranging from parents to preeminent researchers and leaders. Before coming to Nationwide Children’s, Abbie used her communication skills to engage audiences with a wide variety of science topics. She is a Medical Writer Certified®, credentialed by the American Medical Writers Association.
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
- Post Tags:
- Center for Microbial Pathogenesis