A Narrow Focus: Perfecting Tissue Engineered Vascular Grafts
A Narrow Focus: Perfecting Tissue Engineered Vascular Grafts https://pediatricsnationwide.org/wp-content/uploads/2017/04/Annual-Report-Tissue-Engineering_Big-header-1024x575.gif 1024 575 Abbie Miller Abbie Miller https://pediatricsnationwide.org/wp-content/uploads/2023/05/051023BT016-Abbie-Crop.jpg- April 24, 2017
- Abbie Miller
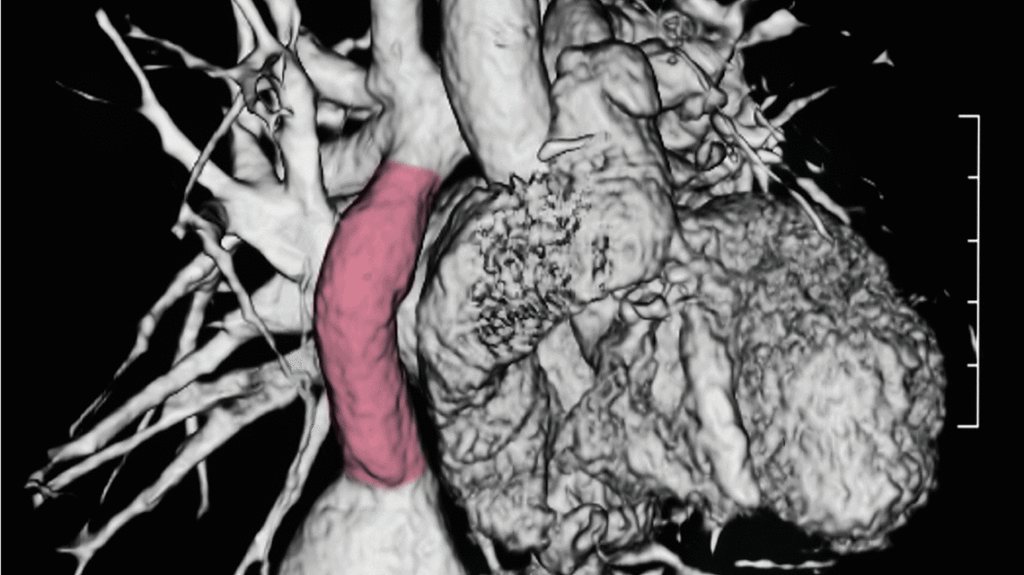
“I think the notion in translational medicine of going from bench to bedside is a little bit flawed,” explains Christopher Breuer, MD, pediatric surgeon and principal investigator at Nationwide Children’s Hospital. “In that any time you start to do something in humans, you’re really starting all over again at some level.”
For the last 23 years Dr. Breuer and Toshiharu Shinoka, MD, PhD, co-directors of the Tissue Engineering Program in The Research Institute at Nationwide Children’s, have been working together to use tissue engineering techniques in congenital heart surgery. Shortly after they met at Harvard University in 1994, they developed one of the first tissue engineered blood vessels and the first tissue engineered valve.
“We were very fortunate,” says Dr. Breuer. “Usually, when you try things in the laboratory they fail more often than they succeed. We were able to create functional valves and vessels in only about a year’s worth of work.”
Fast forward to Yale University in 2012. Drs. Breuer and Shinoka implanted the first tissue engineered vascular graft in a human patient in the United States as part of a landmark clinical trial just before moving to Nationwide Children’s. Since then, Dr. Shinoka and his surgical colleagues at Nationwide Children’s have implanted three additional tissue engineered vascular grafts (TEVGs) in children with single ventricle (SV) congenital heart anomalies.
“Tissue engineered vascular grafts are superior to other options for pediatric congenital heart patients for several reasons, the most important of which is the graft’s growth capacity,” Dr. Shinoka says. “Our grafts don’t require immunosuppression or anti-rejection medications because they are made up of the patient’s own cells. And they grow with the child, decreasing the number of follow-up surgeries needed with conventional grafts.”
One might think the innovation stops here: the team has a working graft with growth potential in a clinical trial. But for Drs. Breuer and Shinoka, the successes in the clinic drive the bench work.
“What we learn from our patients in the clinical study is incredibly important to the bench work. While you can certainly learn a lot in theoretical and animal studies, when you get to the clinic, there’s always a bit of fine tuning, some sense of starting over,” Dr. Breuer says. “The ability to go back and forth between the lab and clinic is vital to our process. And that’s probably the biggest distinction of our laboratory; it all sort of blends together into one project.”
Drs. Breuer and Shinoka have been partners in the lab and the operating room for more than two decades. As co-directors of the Tissue Engineering Program, they lead a diverse team of scientists and surgeons in tissue engineering research spanning cardiothoracic surgery, general pediatric surgery and otolaryngology applications.

The Right Thing for the Wrong Reason
Through the combination of clinical studies and continued bench work, Drs. Breuer and Shinoka have discovered important aspects about how TEVGs respond in a human body. And they have identified the leading complication associated with TEVGs: stenosis.
After the Fontan procedure – the surgery in which the TEVG is placed – the blood flows from the heart to the body and then back to the lungs. It is oxygenated passively, not pumped through the lungs as in normal circulation.
“If narrowing of the graft occurs, less blood will flow to the lungs to be oxygenated, resulting in patients who do more poorly and have worse exercise tolerance. Having a widely open blood vessel is critical to these patients,” says Dr. Shinoka. “It’s the difference between children playing outside at recess or sitting on the sidelines, or worse yet, waiting for another surgery.”
The team has spent 14 years studying the causes of stenosis, including the development of a mouse model.
“It might seem counterintuitive to make grafts for mice when you are already doing grafts in the clinic,” says Dr. Breuer. “But there are tools available for studying mice that are simply not available in other species.”
These tools and the continued bench work led to the initial discovery that they were doing the right things for the wrong reasons.
Briefly, the process of creating and implanting a TEVG involves: placing the scaffold in a vacuum, seeding it with cells obtained from the patient’s bone marrow at the beginning of surgery and then placing the graft. Over the next six months, the body acts as a bioreactor to grow a new vessel. The scaffold disintegrates. When they started, they assumed that the cells that were seeded onto the scaffold made the resulting vessel, but surprisingly, they found the host cells were the ones that made the vessel.
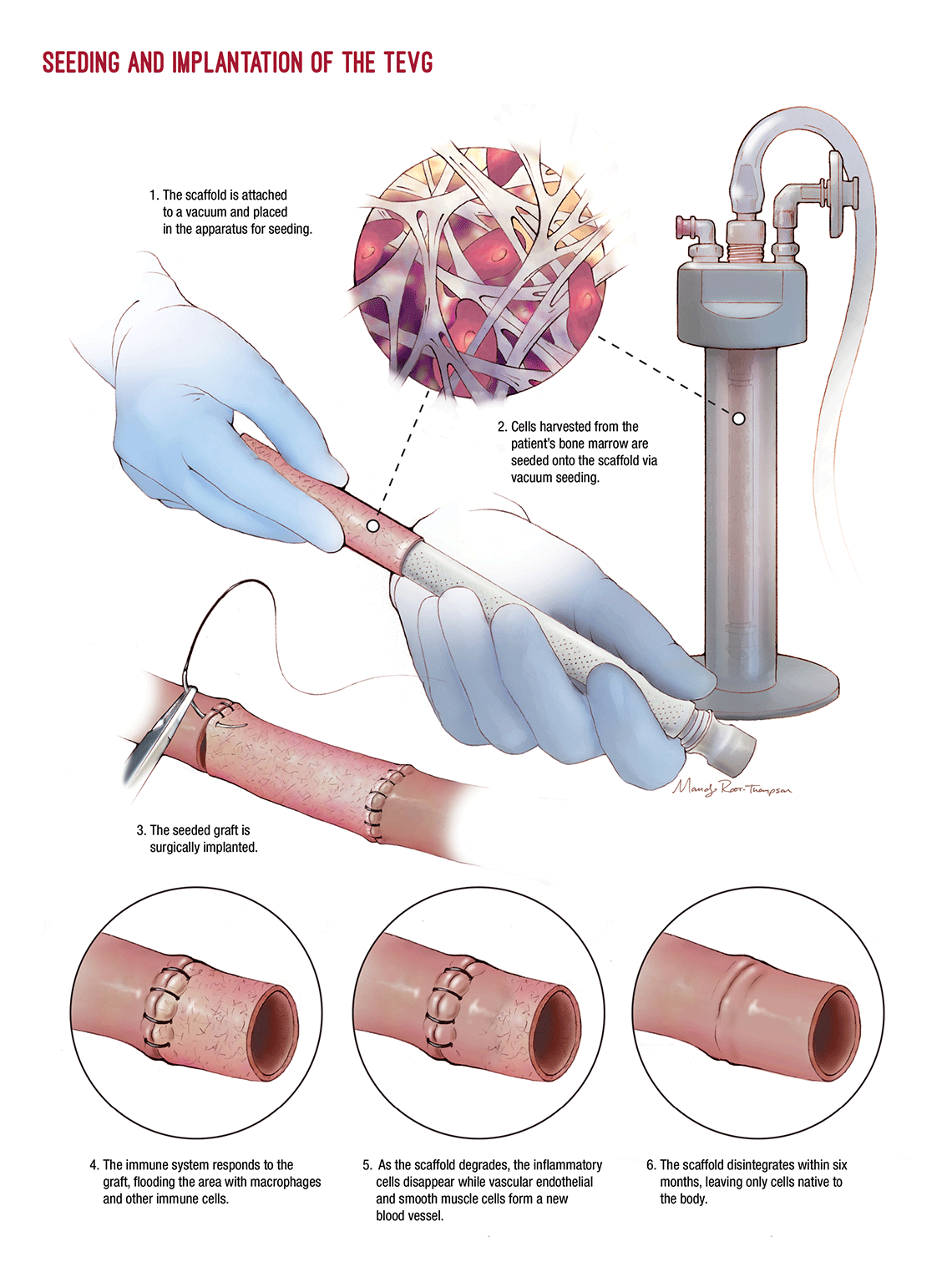
In fact, Dr. Breuer explains, “We discovered that you could make the vessels without seeding the scaffolds, but they didn’t work as well and were more prone to stenosis. Our later work shows a correlation between stenosis and the number of cells seeded on the scaffold.”
The scaffold is essential, but once it is in place, the body takes over and runs the show. According to Drs. Breuer and Shinoka, the cells that are the most important to the whole process are the immune cells, particularly macrophages.
“Macrophages orchestrate the whole process,” says Dr. Breuer. “If you can control them, you can control vascular graft formation. It’s all connected. The scaffold causes a host response. The physical and chemical properties of the scaffold affect the macrophages. The cell seeding affects the properties of the scaffold.”
Blocking Stenosis
In addition to understanding every component of the TEVG process, Dr. Breuer and his team have delved into learning exactly how stenosis forms.
Transforming growth factor beta (TGF-β) is a cytokine that is involved in many cellular functions, including the regulation of cell growth, proliferation, differentiation and apoptosis. It turns out, TGF-β is also integral in the formation of stenosis.
“If you block the receptor for TGF-β, you can block stenosis,” Dr. Breuer says.
Another line of inquiry led to a serendipitous genetic finding. Some mice with certain mutations rarely form stenoses.
While working with mouse models, the team placed TEVGs in SCID beige mice. SCID is a mutation that essentially eliminates the immune system of the mouse. The human equivalent to the beige gene is lysosomal transport gene (LYST), which is an intracellular protein. Mutations of LYST in humans result in Chediak-Higashi syndrome, which affects many parts of the body, particularly the immune system.
“The more we used the model, the more frustrated we became. The grafts formed really well, but the stenosis I was trying to study almost never occurred,” explains Dr. Breuer. “Finally I thought, ‘maybe this isn’t the problem, maybe this is the solution.’ You see, it turns out that the beige mutation is very important for grafts. If you put grafts in beige mice, stenosis almost never forms. Now we’re looking at how to block the beige gene, possibly with an antibody against beige protein.”
Refining the Bedside Approach
All of this research has led to five promising interventions that Drs. Breuer and Shinoka hope to incorporate in their clinical trial. They have submitted their data and are working with the FDA to gain approval to begin the next phase of the trial.
First, the team proposes to seed more cells on the graft before implantation. “This is fairly easy to do,” says Dr. Breuer. “And we can mathematically calculate and analyze risks and benefits of altering the amount of cells seeded to reach optimization.”
Secondly, they plan to address the TGF-β pathway using medications. Losartan is a clinically available medication that is already FDA approved for this patient population. The investigational drug SB431542 is still in development and, while promising, is a ways off from FDA-approval. Both drugs show promise in preventing stenosis by altering the TGF-β pathway, according to Dr. Breuer.
“The investigational drug works really well in the models,” says Dr. Breuer. “But losartan is already approved and safe for this population. We’ve included both drugs in our petition to the FDA, but getting losartan to the clinic for the trial is likely to happen more quickly and with less risk.”
Another promising drug is cilastazol, which does not target the TGF-β pathway. Instead, it targets platelets. Cilastazol is FDA-approved for adults with risk of stenosis, but it is not yet approved for use in children.
Finally, the team is working toward use of an anti-beige antibody to block the beige protein, mimicking the effect of beige genomics on the graft in the mouse model.
Perfecting the Scaffold
In the meantime, the team continues to refine the scaffold.
“We know how important the chemical and physical properties of the scaffold are to the whole process and to how much the graft does or does not become stenotic,” says Dr. Breuer. “Small adjustments to the scaffold have the potential to totally stop stenosis.”
With an infinite number of ways to alter its design and structure, they looked to computational modeling and engineering principles to narrow the scope of possibilities. They partnered with Jay Humphrey, PhD, John C. Malone Professor of Biomedical Engineering and chair, Yale University School of Engineering and Applied Science, to begin a computational modeling project to describe tissue formation in the vascular graft and the body’s response to the scaffold.
“You first have to understand what’s happening when everything goes well,” says Dr. Humphrey. “We do this by building models of the truly successful grafts, ones without stenosis.”
These models will direct the future research projects to refine the design of the scaffold.
“By taking data points that are limited and finding the functional relationships that capture those data points – that describe or reflect them – we can build models that are predictive,” explains Dr. Humphrey. “Once a model is validated, we can, with confidence, predict things that haven’t been observed or measured.”
This predictive power has the potential to move the research at a remarkable pace.
“Instead of doing a million experiments, we are letting the computers do some of the heavy lifting,” says Dr. Breuer. “Then we can focus on the handful of experiments that we hope will get us to the desired result.”
With a shared interest in applying engineering principles to understanding vascular function, Drs. Breuer, Shinoka and Humphrey are exploring the possibilities. By going full circle to advance the science, which ultimately advances the patient care by leaps and bounds, the team understands that the process of discovery, clinical implementation and success is a two-way street. Success is redefined by each new discovery.
“Our clinical experience with TEVGs has laid the foundation for extensive research toward the development of the ideal scaffold,” says Dr. Breuer. “And our efforts to discover interventions against stenosis and optimize the scaffold design are rapidly approaching clinical translation. This is the essence of translational medicine and the hope for curing congenital heart disease.”
References:
- Best C, Onwuka E, Pepper V, Sams M, Breuer J, Breuer C. Cardiovascular tissue engineering: Preclinical validation to bedside application. Physiology. 2016 Jan;31(1):7-15.
- Hibino N, Mejias D, Pietris N, Dean E, Yi T, Shinoka T, Breuer C. The innate immune system contributes to tissue-engineered vascular graft performance. FASEB Journal. 2015 Jun;29(6):2431-2438.
- Lee YU, de Dios Ruiz-Rosado J, Mahler N, Best CA, Tara S, Yi T, Shoji T, Sugiura T, Lee AY, Robledo-Avila F, Hibino N, Pober JS, Shinoka T, Partida-Sanchez S, Breuer CK. TGF-β receptor 1 inhibition prevents stenosis of tissue engineered vascular grafts by reducing host mononuclear phagocyte activation. FASEB Journal. 2016 Jul;30(7):2627-2636.
- Miller KS, Khosravi R, Breuer CK, Humphrey JD. A hypothesis-driven parametric study of effects of polymeric scaffold properties on tissue engineered neovessel formation. Acta Biomaterials. 2015 Jan;11:283-294.
- Tara S, Kurobe H, de Dios Ruiz-Rosado J, Best CA, Shoji T, Mahler N, Yi T, Lee YU, Sugiura T, Hibino N, Partida-Sanchez S, Breuer CK, Shinoka T. Cilostazol, not aspirin, prevents stenosis of bioresorbable vascular grafts in a venous model. Atherosclerosis, Thrombosis and Vascular Biology. 2015 Sep;35(9):2003-2010.
Image credits: Nationwide Children’s
About the author
Abbie (Roth) Miller, MWC, is a passionate communicator of science. As the manager, medical and science content, at Nationwide Children’s Hospital, she shares stories about innovative research and discovery with audiences ranging from parents to preeminent researchers and leaders. Before coming to Nationwide Children’s, Abbie used her communication skills to engage audiences with a wide variety of science topics. She is a Medical Writer Certified®, credentialed by the American Medical Writers Association.
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/
-
Abbie Millerhttps://pediatricsnationwide.org/author/abbie-miller/





